|
Auxochrome
An auxochrome (from Ancient Greek ''auxanō'' "increase" and ''chrōma'' "colour") is a group of atoms attached to a chromophore which modifies the ability of that chromophore to absorb light. They themselves fail to produce the colour; but when present along with the chromophores in an organic compound intensifies the colour of the chromogen. Examples include the hydroxyl group (−OH), the amino group (−NH2), the aldehyde group (−CHO), and the methyl mercaptan group (−SCH3). An auxochrome is a functional group of atoms with one or more lone pairs of electrons when attached to a chromophore, alters both the wavelength and intensity of absorption. If these groups are in direct conjugation with the pi-system of the chromophore, they may increase the wavelength at which the light is absorbed and as a result intensify the absorption. A feature of these auxochromes is the presence of at least one lone pair of electrons which can be viewed as extending the conjugated system by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Woodward's Rules
Woodward's rules, named after Robert Burns Woodward and also known as Woodward–Fieser rules (for Louis Fieser) are several sets of empirically derived rules which attempt to predict the wavelength of the absorption maximum (λmax) in an ultraviolet–visible spectrum of a given compound. Inputs used in the calculation are the type of chromophores present, the auxochromes (substituents on the chromophores, and solvent. Examples are conjugated carbonyl compounds, conjugated dienes, and polyenes. Implementation One set of Woodward–Fieser rules for diene In organic chemistry a diene ( ) (diolefin ( ) or alkadiene) is a covalent compound that contains two double bonds, usually among carbon atoms. They thus contain two alk''ene'' units, with the standard prefix ''di'' of systematic nomenclature. ...s is outlined in table 1. A diene is either homoannular with both double bonds contained in one ring or heteroannular with two double bonds distributed between two rings. With t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromophore
A chromophore is the part of a molecule responsible for its color. The color that is seen by our eyes is the one not absorbed by the reflecting object within a certain wavelength spectrum of visible light. The chromophore is a region in the molecule where the energy difference between two separate molecular orbitals falls within the range of the visible spectrum. Visible light that hits the chromophore can thus be absorbed by exciting an electron from its ground state into an excited state. In biological molecules that serve to capture or detect light energy, the chromophore is the moiety that causes a conformational change in the molecule when hit by light. Conjugated pi-bond system chromophores Just like how two adjacent p-orbitals in a molecule will form a pi-bond, three or more adjacent p-orbitals in a molecule can form a conjugated pi-system. In a conjugated pi-system, electrons are able to capture certain photons as the electrons resonate along a certain distance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ancient Greek Language
Ancient Greek includes the forms of the Greek language used in ancient Greece and the classical antiquity, ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Greek Dark Ages, Dark Ages (), the Archaic Greece, Archaic period (), and the Classical Greece, Classical period (). Ancient Greek was the language of Homer and of fifth-century Athens, fifth-century Athenian historians, playwrights, and Ancient Greek philosophy, philosophers. It has contributed many words to English vocabulary and has been a standard subject of study in educational institutions of the Western world since the Renaissance. This article primarily contains information about the Homeric Greek, Epic and Classical periods of the language. From the Hellenistic period (), Ancient Greek was followed by Koine Greek, which is regarded as a separate historical stage, although its earliest form closely resembles Attic Greek and its latest form a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillating force is applied at a resonant frequency of a dynamic system, the system will oscillate at a higher amplitude than when the same force is applied at other, non-resonant frequencies. Frequencies at which the response amplitude is a relative maximum are also known as resonant frequencies or resonance frequencies of the system. Small periodic forces that are near a resonant frequency of the system have the ability to produce large amplitude oscillations in the system due to the storage of vibrational energy. Resonance phenomena occur with all types of vibrations or waves: there is mechanical resonance, orbital resonance, acoustic resonance, electromagnetic resonance, nuclear magnetic resonance (NMR), electron spin resonance (ESR) and reso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
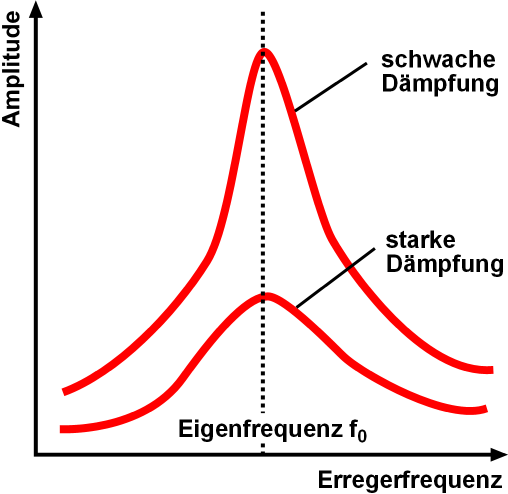
Natural Frequency
Natural frequency, also known as eigenfrequency, is the frequency at which a system tends to oscillate in the absence of any driving force. The motion pattern of a system oscillating at its natural frequency is called the normal mode (if all parts of the system move sinusoidally with that same frequency). If the oscillating system is driven by an external force at the frequency at which the amplitude of its motion is greatest (close to a natural frequency of the system), this frequency is called resonant frequency. Overview Free vibrations of an elastic body are called ''natural vibrations'' and occur at a frequency called the natural frequency. Natural vibrations are different from forced vibrations which happen at the frequency of an applied force (forced frequency). If the forced frequency is equal to the natural frequency, the vibrations' amplitude increases manyfold. This phenomenon is known as resonance. In a mass-spring system, with mass ''m'' and spring stiffness ' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a sequence of database operations that satisfies the ACID properties (which can be perceived as a single logical operation on the data) is called a ''transaction''. For example, a transfer of funds from one bank account to another, even involving multiple changes such as debiting one account and crediting another, is a single transaction. In 1983, Andreas Reuter and Theo Härder coined the acronym ''ACID'', building on earlier work by Jim Gray who named atomicity, consistency, and durability, but not isolation, when characterizing the transaction concept. These four properties are the major guarantees of the transaction paradigm, which has influenced many aspects of development in database systems. According to Gray and Reuter, the IBM Informa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction An acid–base reaction is a chemical reaction that occurs between an acid and a base. It can be used to determine pH via titration. Several theoretical frameworks provide alternative conceptions of the reaction mechanisms and their applica .... A base was therefore a metal hydroxide such as Sodium hydroxide, NaOH or Calcium hydroxide, Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolytic Dissociation
An electrolyte is a medium containing ions that is electrically conducting through the movement of those ions, but not conducting electrons. This includes most soluble salts, acids, and bases dissolved in a polar solvent, such as water. Upon dissolving, the substance separates into cations and anions, which disperse uniformly throughout the solvent. Solid-state electrolytes also exist. In medicine and sometimes in chemistry, the term electrolyte refers to the substance that is dissolved. Electrically, such a solution is neutral. If an electric potential is applied to such a solution, the cations of the solution are drawn to the electrode that has an abundance of electrons, while the anions are drawn to the electrode that has a deficit of electrons. The movement of anions and cations in opposite directions within the solution amounts to a current. Some gases, such as hydrogen chloride (HCl), under conditions of high temperature or low pressure can also function as electrolytes. Ele ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugated System
In theoretical chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally represented as having alternating single and multiple bonds. Lone pairs, radicals or carbenium ions may be part of the system, which may be cyclic, acyclic, linear or mixed. The term "conjugated" was coined in 1899 by the German chemist Johannes Thiele. Conjugation is the overlap of one p-orbital with another across an adjacent σ bond (in transition metals, d-orbitals can be involved). A conjugated system has a region of overlapping p-orbitals, bridging the interjacent locations that simple diagrams illustrate as not having a π bond. They allow a delocalization of π electrons across all the adjacent aligned p-orbitals. The π electrons do not belong to a single bond or atom, but rather to a group of atoms. Molecules containing conjugated syst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infrared Light
Infrared (IR), sometimes called infrared light, is electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye. IR is generally understood to encompass wavelengths from around 1 millimeter (300 GHz) to the nominal red edge of the visible spectrum, around 700 nanometers (430 THz). Longer IR wavelengths (30 μm-100 μm) are sometimes included as part of the terahertz radiation range. Almost all black-body radiation from objects near room temperature is at infrared wavelengths. As a form of electromagnetic radiation, IR propagates energy and momentum, exerts radiation pressure, and has properties corresponding to both those of a wave and of a particle, the photon. It was long known that fires emit invisible heat; in 1681 the pioneering experimenter Edme Mariotte showed that glass, though transparent to sunlight, obstructed radiant heat. In 1800 the astronomer Sir William Herschel discovered ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bathochromic Shift
Bathochromic shift (from Greek βαθύς ''bathys'', "deep"; and χρῶμα ''chrōma'', "color"; hence less common alternate spelling "bathychromic") is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength (lower frequency). Because the red color in the visible spectrum has a longer wavelength than most other colors, the effect is also commonly called a '' red shift''. Hypsochromic shift is a change to shorter wavelength (higher frequency). Conditions It can occur because of a change in environmental conditions: for example, a change in solvent polarity will result in solvatochromism. A series of structurally-related molecules in a substitution series can also show a bathochromic shift. Bathochromic shift is a phenomenon seen in ''molecular'' spectra, not ''atomic'' spectra; it is thus more common to speak of the movement of the peaks in the spectrum rather than lines. :\Delta\lambda = \la ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpeg/1200px-Fall_Leaves_(199582361).jpeg)