|
Antibody–drug Conjugate
Antibody–drug conjugates or ADCs are a class of biopharmaceutical drugs designed as a targeted therapy for treating cancer. Unlike chemotherapy, ADCs are intended to target and kill tumor cells while sparing healthy cells. As of 2019, some 56 pharmaceutical companies were developing ADCs. ADCs are complex molecules composed of an antibody linked to a biologically active cytotoxic (anticancer) payload or drug. Antibody–drug conjugates are an example of bioconjugates and immunoconjugates. ADCs combine the targeting properties of monoclonal antibodies with the cancer-killing capabilities of cytotoxic drugs, designed to discriminate between healthy and diseased tissue. Mechanism of action An anticancer drug is coupled to an antibody that targets a specific tumor antigen (or protein) that, ideally, is only found in or on tumor cells. Antibodies attach themselves to the antigens on the surface of cancerous cells. The biochemical reaction that occurs upon attaching triggers a si ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accelerated Approval (FDA)
The United States Food and Drug Administration (FDA) initiated the FDA Accelerated Approval Program in 1992 to allow faster approval of drugs for serious conditions that fill an unmet medical need. The faster approval relies on use of surrogate endpoints. Drug approval typically requires clinical trials with endpoints that demonstrate a clinical benefit, such as increased survival for cancer patients. Drugs with accelerated approval can initially be tested in clinical trials that use a surrogate endpoint, or something that is thought to predict clinical benefit. Surrogate endpoints typically require less time, and in the case of a cancer patient, it is much faster to measure a reduction in tumor size, for example, than overall patient survival. Drugs approved under the FDA Accelerated Approval Program still need to be tested in clinical trials using endpoints that demonstrate clinical benefit, and those trials are known as phase 4 confirmatory trials. If the drug later proves unabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Subcutaneous Injection
Subcutaneous administration is the insertion of medications beneath the skin either by injection or infusion. A subcutaneous injection is administered as a bolus (medicine), bolus into the subcutis, the layer of skin directly below the dermis and Epidermis (skin), epidermis, collectively referred to as the Cutis (anatomy), cutis. The instruments are usually a hypodermic needle and a syringe. Subcutaneous injections are highly effective in administering medications such as insulin, morphine, heroin, diacetylmorphine and goserelin. Subcutaneous administration may be List of medical abbreviations, abbreviated as SC, SQ, subcu, sub-Q, SubQ, or subcut. Subcut is the preferred abbreviation to reduce the risk of misunderstanding and potential errors. Subcutaneous tissue has few blood vessels and so drugs injected into it are intended for slow, sustained rates of absorption, often with some amount of depot injection, depot effect. Compared with other route of administration, routes of ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
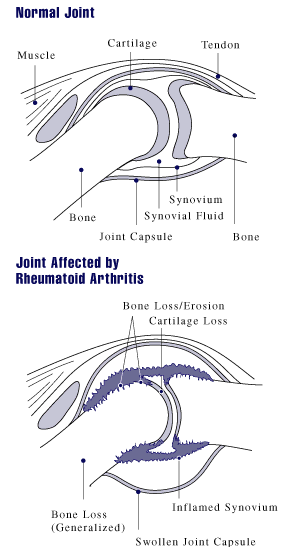
Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects synovial joint, joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and hands are involved, with the same joints typically involved on both sides of the body. The disease may also affect other parts of the body, including skin, eyes, lungs, heart, nerves, and blood. This may result in a anemia, low red blood cell count, pleurisy, inflammation around the lungs, and pericarditis, inflammation around the heart. Fever and low energy may also be present. Often, symptoms come on gradually over weeks to months. While the cause of rheumatoid arthritis is not clear, it is believed to involve a combination of genetic and environmental factors. The underlying mechanism involves the body's immune system attacking the joints. This results in inflammation and thickening of the synovium, joint capsule. It also affects the und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Immunology
Immunology is a branch of biology and medicine that covers the study of Immune system, immune systems in all Organism, organisms. Immunology charts, measures, and contextualizes the Physiology, physiological functioning of the immune system in states of both health and diseases; malfunctions of the immune system in immunological disorders (such as Autoimmune disease, autoimmune diseases, Hypersensitivity, hypersensitivities, immune deficiency, and transplant rejection); and the physical, chemical, and physiological characteristics of the components of the immune system ''in vitro'', ''In situ#Biology and biomedical engineering, in situ'', and ''in vivo''. Immunology has applications in numerous disciplines of medicine, particularly in the fields of organ transplantation, oncology, rheumatology, virology, bacteriology, parasitology, psychiatry, and dermatology. The term was coined by Russian biologist Ilya Ilyich Mechnikov, who advanced studies on immunology and received the Nob ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acute Lymphoblastic Leukemia
Acute lymphoblastic leukemia (ALL) is a cancer of the Lymphocyte, lymphoid line of blood cells characterized by the development of large numbers of lymphoblast, immature lymphocytes. Symptoms may include feeling tired, pale skin color, fever, easy bleeding or bruising, lymphadenopathy, enlarged lymph nodes, or bone pain. As an acute leukemia, ALL progresses rapidly and is typically fatal within weeks or months if left untreated. In most cases, the cause is unknown. Genetic risk factors may include Down syndrome, Li–Fraumeni syndrome, or neurofibromatosis type 1. Environmental risk factors may include significant radiation exposure or prior chemotherapy. Evidence regarding electromagnetic fields or pesticides is unclear. Some hypothesize that an abnormal immune response to a common infection may be a trigger. The underlying mechanism involves multiple genetic mutations that results in rapid cell division. The excessive immature lymphocytes in the bone marrow interfere with t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inotuzumab Ozogamicin
Inotuzumab ozogamicin, sold under the brand name Besponsa, is an antibody-drug conjugate medication used to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL). It is administered by intravenous infusion. Inotuzumab ozogamicin consists of a humanized monoclonal antibody against CD22 ( inotuzumab), linked to a cytotoxic agent from the class of calicheamicins called ozogamicin. The US Food and Drug Administration considers it to be a first-in-class medication. Medical use Inotuzumab ozogamicin is used to treat relapsed or refractory B-cell precursor acute lymphoblastic leukemia. In March 2024, the US Food and Drug Administration approved inotuzumab ozogamicin for the treatment of children aged one year and older with relapsed or refractory CD22-positive B-cell precursor acute lymphoblastic leukemia. Adverse effects The US Food and Drug Administration label for the use of inotuzumab ozagamicin carries a boxed warning concerning the risk of liv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Commission
The European Commission (EC) is the primary Executive (government), executive arm of the European Union (EU). It operates as a cabinet government, with a number of European Commissioner, members of the Commission (directorial system, informally known as "commissioners") corresponding to two thirds of the number of Member state of the European Union, member states, unless the European Council, acting unanimously, decides to alter this number. The current number of commissioners is 27, including the president. It includes an administrative body of about 32,000 European civil servants. The commission is divided into departments known as Directorate-General, Directorates-General (DGs) that can be likened to departments or Ministry (government department), ministries each headed by a director-general who is responsible to a commissioner. Currently, there is one member per European Union member state, member state, but members are bound by their oath of office to represent the genera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Taxane
Taxanes are a class of diterpenes. They were originally identified from plants of the genus ''Taxus'' (yews), and feature a taxadiene core. Paclitaxel (Taxol) and docetaxel (Taxotere) are widely used as chemotherapy agents. Cabazitaxel was FDA approved to treat hormone-refractory prostate cancer. Taxanes present difficulties in Pharmaceutical formulation, formulation as medicines because they are poorly soluble in water. Production As their name suggests, taxanes were first derived from natural sources, but some have been semisynthesis, semisynthesized. Paclitaxel was originally derived from the Pacific yew tree. Taxanes are difficult to synthesize because of their numerous chiral centres—taxol has 11 of these. Recently, the presence of taxanes in the shells and leaves of ''Corylus avellana'' (the common hazel plant) has been reported. Mechanism of action The principal mechanism of action of the taxane class of drugs is the disruption of microtubule function. Microtubule ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HER2/neu
Receptor tyrosine-protein kinase erbB-2 is a protein that normally resides in the membranes of cells and is encoded by the ''ERBB2'' gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The human protein is also frequently referred to as HER2 (human epidermal growth factor receptor 2) or CD340 ( cluster of differentiation 340). HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family. But contrary to other members of the ERBB family, HER2 does not directly bind ligand. HER2 activation results from heterodimerization with another ERBB member or by homodimerization when HER2 concentration are high, for instance in cancer. Amplification or over-expression of this oncogene has been shown to play an important role in the development and progression of certain aggressive types of breast cancer. In recent years the protein has become an important biomarker and target of therapy for approximately 30% ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trastuzumab Emtansine
Trastuzumab emtansine, sold under the brand name Kadcyla, is an antibody-drug conjugate consisting of the humanized monoclonal antibody trastuzumab (Herceptin) covalently linked to the cytotoxic agent DM1. Trastuzumab alone stops growth of cancer cells by binding to the HER2 receptor, whereas trastuzumab emtansine undergoes receptor-mediated internalization into cells, is catabolized in lysosomes where DM1-containing catabolites are released and subsequently bind tubulin to cause mitotic arrest and cell death. Trastuzumab binding to HER2 prevents homodimerization or heterodimerization (HER2/HER3) of the receptor, ultimately inhibiting the activation of MAPK and PI3K/AKT cellular signalling pathways. Because the monoclonal antibody targets HER2, and HER2 is only over-expressed in cancer cells, the conjugate delivers the cytotoxic agent DM1 specifically to tumor cells. The conjugate is abbreviated T-DM1. In the EMILIA clinical trial of women with advanced HER2 positive breas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of pharmaceutical products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |