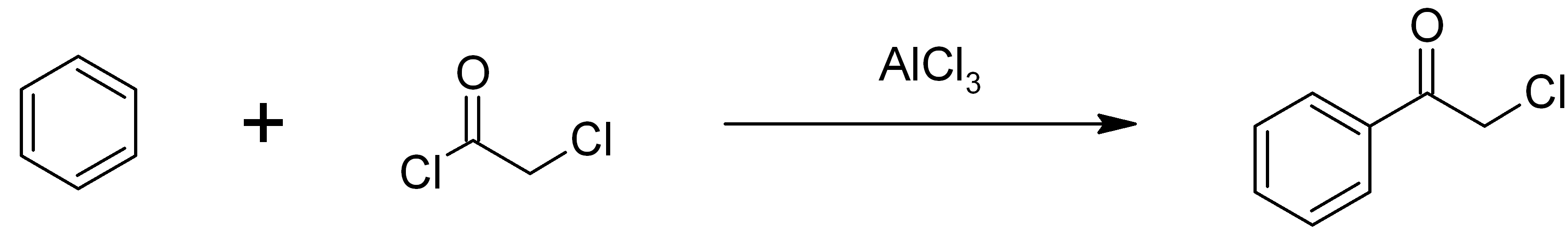|
Amidoxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides () with general structure . Oximes are usually generated by the reaction of hydroxylamine with aldehydes () or ketones (). The term ''oxime'' dates back to the 19th century, a combination of the words ''oxygen'' and ''imine''. Structure and properties If the two side-chains on the central carbon are different from each other—either an aldoxime, or a ketoxime with two different "R" groups—the oxime can often have two different geometric stereoisomeric forms according to the ''E''/''Z'' configuration. An older terminology of ''syn'' and ''anti'' was used to identify especially aldoximes according to whether the R group was closer or further from th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
General Structure Of Oximes
A general officer is an officer of high rank in the armies, and in some nations' air forces, space forces, and marines or naval infantry. In some usages the term "general officer" refers to a rank above colonel."general, adj. and n.". OED Online. March 2021. Oxford University Press. https://www.oed.com/view/Entry/77489?rskey=dCKrg4&result=1 (accessed May 11, 2021) The term ''general'' is used in two ways: as the generic title for all grades of general officer and as a specific rank. It originates in the 16th century, as a shortening of ''captain general'', which rank was taken from Middle French ''capitaine général''. The adjective ''general'' had been affixed to officer designations since the late medieval period to indicate relative superiority or an extended jurisdiction. Today, the title of ''general'' is known in some countries as a four-star rank. However, different countries use different systems of stars or other insignia for senior ranks. It has a NATO rank scal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michigan State University
Michigan State University (Michigan State, MSU) is a public university, public Land-grant university, land-grant research university in East Lansing, Michigan. It was founded in 1855 as the Agricultural College of the State of Michigan, the first of its kind in the United States. It is considered a Public Ivy, or a public institution which offers an academic experience similar to that of an Ivy League university. After the introduction of the Morrill Land-Grant Acts, Morrill Act in 1862, the state designated the college a land-grant institution in 1863, making it the first of the land-grant colleges in the United States. The college became coeducational in 1870. In 1955, the state officially made the college a university, and the current name, Michigan State University, was adopted in 1964. Today, Michigan State has the largest undergraduate enrollment among Michigan's colleges and universities and approximately 634,300 living alums worldwide. The university is a member of the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Malononitrile
Malononitrile is an organic compound nitrile with the formula . It is a colorless or white solid. It can be prepared by dehydration of cyanoacetamide. Malononitrile is relatively acidic, with a p''K''a of 11 in water. This allows it to be used in the Knoevenagel condensation, for example in the preparation of CS gas: Malononitrile is a suitable starting material for the Gewald reaction, where the nitrile condenses with a ketone or aldehyde in the presence of elemental sulfur and a base to produce a 2-aminothiophene. See also * Malonic acid * Diethyl malonate Diethyl malonate, also known as DEM, is the diethyl ester of malonic acid. It occurs naturally in grapes and strawberries as a colourless liquid with an apple-like odour, and is used in perfumes. It is also used to synthesize other compounds su ... References External links WebBook page for C3H2N2 {{Authority control Alkanedinitriles ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenacyl Chloride
Phenacyl chloride, also commonly known as chloroacetophenone, is a substituted acetophenone. It is a useful building block in organic chemistry. Apart from that, it has been historically used as a riot control agent, where it is designated CN. It should not be confused with cyanide, another agent used in chemical warfare, which has the chemical structure CN−. Preparation Phenacyl chloride is readily available and was first prepared by chlorination of acetophenone vapour. It may also be synthesized by the Friedel-Crafts acylation of benzene using chloroacetyl chloride, with an aluminium chloride catalyst: Riot control agent It was investigated, but not used, during the First and Second World Wars. Because of its significantly greater toxicity, it has largely been supplanted by CS gas. Even though CN is still supplied to paramilitary and police forces in a small pressurized aerosol known as “ Mace” or tear gas, its use is falling as pepper spray both works and disperses mor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propiophenone
Propiophenone (shorthand: benzoylethane or BzEt) is an aryl ketone. It is a colorless, sweet-smelling liquid that is insoluble in water, but miscible with organic solvents. It is used in the preparation of other compounds. Production Propiophenone can be prepared by Friedel–Crafts reaction of propanoyl chloride and benzene. It is also prepared commercially by ketonization of benzoic acid and propionic acid over calcium acetate Calcium acetate is a chemical compound which is a calcium salt of acetic acid. It has the formula Ca(C2H3O2)2. Its standard name is calcium acetate, while calcium ethanoate is the systematic name. An older name is acetate of lime. The anhydrous ... and alumina at 450–550 °C: :C6H5CO2H + CH3CH2CO2H → C6H5C(O)CH2CH3 + CO2 + H2O Ludwig Claisen discovered that α-methoxystyrene forms this compound when heated for an hour at 300 °C (65% yield). Uses 120px, left, Phenmetrazine, derived from propiophenone, is an appetite suppressant."> ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrochloric Acid
Hydrochloric acid, also known as muriatic acid, is an aqueous solution of hydrogen chloride. It is a colorless solution with a distinctive pungent smell. It is classified as a strong acid Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutions .... It is a component of the gastric acid in the digestive systems of most animal species, including humans. Hydrochloric acid is an important laboratory reagent and industrial chemical. History In the early tenth century, the Persian physician and alchemist Abu Bakr al-Razi ( 865–925, Latin: Rhazes) conducted experiments with sal ammoniac (ammonium chloride) and vitriol (hydrated sulfates of various metals), which he distilled together, thus producing the gas hydrogen chloride. In doing so, al-Razi may have stumbled upon a primitive method ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Nitrite
The chemical compound ethyl nitrite is an alkyl nitrite with a chemical formula C2H5NO2. It may be prepared from ethanol. : Uses It is used as a reagent with butanone to yield the dimethylglyoxime end product. Ethyl nitrite is the main ingredient in a traditional ethanol-based South African remedy for colds and flu known as ''Witdulsies'', which is sold in pharmacies. It is known as a traditional Afrikaans remedy; the same remedy is apparently made by the Amish The Amish (; pdc, Amisch; german: link=no, Amische), formally the Old Order Amish, are a group of traditionalist Anabaptist Christian church fellowships with Swiss German and Alsatian origins. They are closely related to Mennonite churches ... in the US. However, FDA has blocked over-the-counter sales of this same remedy, known in the US as ''sweet nitrite'' or ''sweet spirit of nitre'', since 1980. Its use has been associated with fatal methemoglobinemia. Methemoglobinemia is the primary toxic effect of et ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Ethyl Ketone
Butanone, also known as methyl ethyl ketone (MEK), is an organic compound with the formula CH3C(O)CH2CH3. This colourless liquid ketone has a sharp, sweet odor reminiscent of acetone. It is produced industrially on a large scale, but occurs in nature only in trace amounts.Wilhelm Neier, Guenter Strehlke "2-Butanone" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2002. It is partially soluble in water, and is commonly used as an industrial solvent. It is an isomer of another solvent, tetrahydrofuran. Production Butanone may be produced by oxidation of 2-butanol. The dehydrogenation of 2-butanol is catalysed by copper, zinc, or bronze: :CH3CH(OH)CH2CH3 → CH3C(O)CH2CH3 + H2 This is used to produce approximately 700 million kilograms yearly. Other syntheses that have been examined but not implemented include Wacker oxidation of 2-butene and oxidation of isobutylbenzene, which is analogous to the industrial production of acetone. The cumene process ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Nitrite
Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is a white to slightly yellowish crystalline powder that is very soluble in water and is hygroscopic. From an industrial perspective, it is the most important nitrite salt. It is a precursor to a variety of organic compounds, such as pharmaceuticals, dyes, and pesticides, but it is probably best known as a food additive used in processed meats and (in some countries) in fish products. Uses Industrial chemistry The main use of sodium nitrite is for the industrial production of organonitrogen compounds. It is a reagent for conversion of amines into diazo compounds, which are key precursors to many dyes, such as diazo dyes. Nitroso compounds are produced from nitrites. These are used in the rubber industry. It is used in a variety of metallurgical applications, for phosphatizing and detinning. Sodium nitrite is an effective corrosion inhibitor and is used as an additive in industrial greases, as an aq ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethyl Acetoacetate
The organic compound ethyl acetoacetate (EAA) is the ethyl ester of acetoacetic acid. It is a colorless liquid. It is widely used as a chemical intermediate in the production of a wide variety of compounds. It is used as a flavoring for food. Preparation Ethyl acetoacetate is produced industrially by treatment of diketene with ethanol. The preparation of ethyl acetoacetate is a classic laboratory procedure. It is prepared via the Claisen condensation of ethyl acetate. Two moles of ethyl acetate condense to form one mole each of ethyl acetoacetate and ethanol. : Reactivity Acidity Ethyl acetoacetate is diprotic: :CH3C(O)CH2CO2Et + NaH → CH3C(O)CH(Na)CO2Et + H2 :CH3C(O)CH(Na)CO2Et + BuLi → LiCH2C(O)CH(Na)CO2Et + BuH Keto-enol tautomerism Ethyl acetoacetate is subject to keto-enol tautomerism. In the neat liquid at 33 °C, the enol consists of 15% of the total. Multicarbon building block Ethyl acetoacetic acid is a building block in organic synthesis since the protons alp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isoamyl Nitrite
Amyl nitrite is a chemical compound with the formula C5H11ONO. A variety of isomers are known, but they all feature an amyl group attached to the nitrite functional group. The alkyl group is unreactive and the chemical and biological properties are mainly due to the nitrite group. Like other alkyl nitrites, amyl nitrite is bioactive in mammals, being a vasodilator, which is the basis of its use as a prescription medicine. As an inhalant, it also has a psychoactive effect, which has led to its recreational use, with its smell being described as that of old socks or dirty feet. It is also referred to as ''banapple gas''. It was first documented in 1844 and came into medical use in 1867. Uses * Amyl nitrite is employed medically to treat heart diseases as well as angina. * Amyl nitrite is sometimes used as an antidote for cyanide poisoning. It can act as an oxidant, to induce the formation of methemoglobin. Methemoglobin in turn can sequester cyanide as cyanomethemoglobin. * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |