|
Alpha-synuclein
Alpha-synuclein is a protein that, in humans, is encoded by the ''SNCA'' gene. Alpha-synuclein is a neuronal protein that regulates synaptic vesicle trafficking and subsequent neurotransmitter release. It is abundant in the brain, while smaller amounts are found in the heart, muscle and other tissues. In the brain, alpha-synuclein is found mainly in the axon terminals of presynaptic neurons. Within these terminals, alpha-synuclein interacts with phospholipids and proteins. Presynaptic terminals release chemical messengers, called neurotransmitters, from compartments known as synaptic vesicles. The release of neurotransmitters relays signals between neurons and is critical for normal brain function. The human alpha-synuclein protein is made of 140 amino acids. An alpha-synuclein fragment, known as the non- Abeta component (NAC) of Alzheimer's disease amyloid, originally found in an amyloid-enriched fraction, was shown to be a fragment of its precursor protein, NACP. It was late ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synuclein
Synucleins are a family of soluble proteins common to vertebrates, primarily expressed in neural tissue and in certain tumors. The name is a blend of the words "synapse" and "nucleus", as it was first found in the synapses in the electromotor nucleus of the electric ray. Family members The synuclein family includes three known proteins: alpha-synuclein, beta-synuclein, and gamma-synuclein. Interest in the synuclein family began when alpha-synuclein was found to be mutated in several families with autosomal dominant Parkinson's disease. All synucleins have in common a highly conserved alpha-helical lipid-binding motif with similarity to the class-A2 lipid-binding domains of the exchangeable apolipoproteins. Synuclein family members are not found outside vertebrates, although they have some conserved structural similarity with plant 'late-embryo-abundant' proteins. *Alpha-synuclein * Beta-synuclein * Gamma-synuclein Function Normal cellular functions have not been d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intrinsically Disordered Protein
In molecular biology, an intrinsically disordered protein (IDP) is a protein that lacks a fixed or ordered three-dimensional structure, typically in the absence of its macromolecular interaction partners, such as other proteins or RNA. IDPs range from fully unstructured to partially structured and include random coil, molten globule-like aggregates, or flexible linkers in large multi-domain proteins. They are sometimes considered as a separate class of proteins along with globular, fibrous and membrane proteins. IDPs are a very large and functionally important class of proteins and their discovery has disproved the idea that three-dimensional structures of proteins must be fixed to accomplish their biological functions. For example, IDPs have been identified to participate in weak multivalent interactions that are highly cooperative and dynamic, lending them importance in DNA regulation and in cell signaling. Many IDPs can also adopt a fixed three-dimensional structure after bi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid resid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frontal Cortex
The frontal lobe is the largest of the four major lobes of the brain in mammals, and is located at the front of each cerebral hemisphere (in front of the parietal lobe and the temporal lobe). It is parted from the parietal lobe by a groove between tissues called the central sulcus and from the temporal lobe by a deeper groove called the lateral sulcus (Sylvian fissure). The most anterior rounded part of the frontal lobe (though not well-defined) is known as the frontal pole, one of the three poles of the cerebrum. The frontal lobe is covered by the frontal cortex. The frontal cortex includes the premotor cortex, and the primary motor cortex – parts of the motor cortex. The front part of the frontal cortex is covered by the prefrontal cortex. There are four principal gyri in the frontal lobe. The precentral gyrus is directly anterior to the central sulcus, running parallel to it and contains the primary motor cortex, which controls voluntary movements of specific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Dimer
In biochemistry, a protein dimer is a macromolecular complex formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ''dimer'' has roots meaning "two parts", ''di-'' + '' -mer''. A protein dimer is a type of protein quaternary structure. A protein homodimer is formed by two identical proteins. A protein heterodimer is formed by two different proteins. Most protein dimers in biochemistry are not connected by covalent bonds. An example of a non-covalent heterodimer is the enzyme reverse transcriptase, which is composed of two different amino acid chains. An exception is dimers that are linked by disulfide bridges such as the homodimeric protein NEMO. Some proteins contain specialized domains to ensure dimerization (dimerization domains) and specificity. The G protein-coupled cannabinoid receptors have the ability to form both homo- and heterodimers with sev ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Optical Tweezers
Optical tweezers (originally called single-beam gradient force trap) are scientific instruments that use a highly focused laser beam to hold and move microscopic and sub-microscopic objects like atoms, nanoparticles and droplets, in a manner similar to tweezers. If the object is held in air or vacuum without additional support, it can be called optical levitation. The laser light provides an attractive or repulsive force (typically on the order of piconewtons), depending on the relative refractive index between particle and surrounding medium. Levitation is possible if the force of the light counters the force of gravity. The trapped particles are usually micron-sized, or even smaller. Dielectric and absorbing particles can be trapped, too. Optical tweezers are used in biology and medicine (for example to grab and hold a single bacterium, a cell like a sperm cell or a blood cell, or a molecule like DNA), nanoengineering and nanochemistry (to study and build materials fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
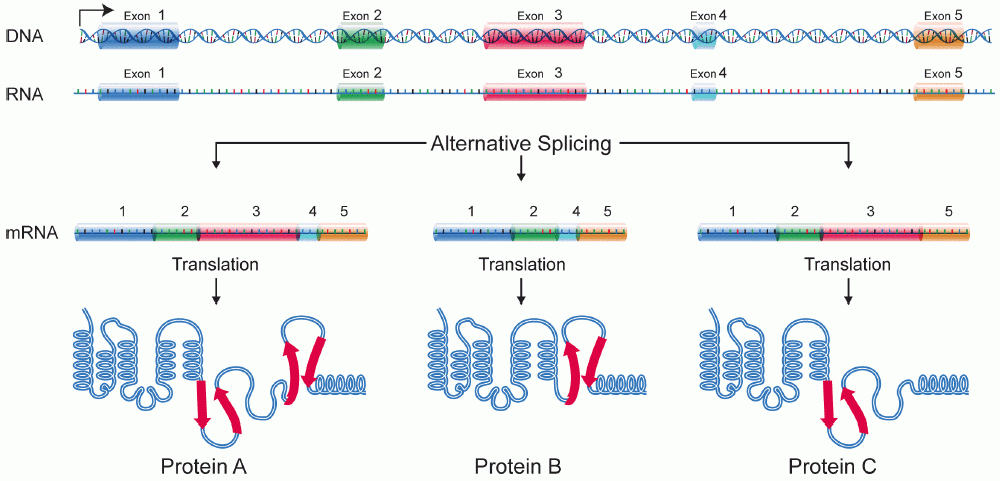
Alternative Splicing
Alternative splicing, or alternative RNA splicing, or differential splicing, is an alternative splicing process during gene expression that allows a single gene to code for multiple proteins. In this process, particular exons of a gene may be included within or excluded from the final, processed messenger RNA (mRNA) produced from that gene. This means the exons are joined in different combinations, leading to different (alternative) mRNA strands. Consequently, the proteins translated from alternatively spliced mRNAs will contain differences in their amino acid sequence and, often, in their biological functions (see Figure). Biologically relevant alternative splicing occurs as a normal phenomenon in eukaryotes, where it increases the number of proteins that can be encoded by the genome. In humans, it is widely believed that ~95% of multi-exonic genes are alternatively spliced to produce functional alternative products from the same gene but many scientists believe that most o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Respiratory Complex I
Respiratory complex I, (also known as NADH:ubiquinone oxidoreductase, Type I NADH dehydrogenase and mitochondrial complex I) is the first large protein complex of the respiratory chains of many organisms from bacteria to humans. It catalyzes the transfer of electrons from NADH to coenzyme Q10 (CoQ10) and translocates protons across the inner mitochondrial membrane in eukaryotes or the plasma membrane of bacteria. This enzyme is essential for the normal functioning of cells, and mutations in its subunits lead to a wide range of inherited neuromuscular and metabolic disorders. Defects in this enzyme are responsible for the development of several pathological processes such as ischemia/reperfusion damage ( stroke and cardiac infarction), Parkinson's disease and others. Function Complex I is the first enzyme of the mitochondrial electron transport chain. There are three energy-transducing enzymes in the electron transport chain - NADH:ubiquinone oxidoreductase (comple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mitochondria
A mitochondrion (; ) is an organelle found in the cells of most Eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is used throughout the cell as a source of chemical energy. They were discovered by Albert von Kölliker in 1857 in the voluntary muscles of insects. The term ''mitochondrion'' was coined by Carl Benda in 1898. The mitochondrion is popularly nicknamed the "powerhouse of the cell", a phrase coined by Philip Siekevitz in a 1957 article of the same name. Some cells in some multicellular organisms lack mitochondria (for example, mature mammalian red blood cells). A large number of unicellular organisms, such as microsporidia, parabasalids and diplomonads, have reduced or transformed their mitochondria into other structures. One eukaryote, '' Monocercomonoides'', is known to have completely lost its mitochondria, and one multicellular orga ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Presynaptic
In the nervous system, a synapse is a structure that permits a neuron (or nerve cell) to pass an electrical or chemical signal to another neuron or to the target effector cell. Synapses are essential to the transmission of nervous impulses from one neuron to another. Neurons are specialized to pass signals to individual target cells, and synapses are the means by which they do so. At a synapse, the plasma membrane of the signal-passing neuron (the ''presynaptic'' neuron) comes into close apposition with the membrane of the target (''postsynaptic'') cell. Both the presynaptic and postsynaptic sites contain extensive arrays of molecular machinery that link the two membranes together and carry out the signaling process. In many synapses, the presynaptic part is located on an axon and the postsynaptic part is located on a dendrite or soma. Astrocytes also exchange information with the synaptic neurons, responding to synaptic activity and, in turn, regulating neurotransmission. Synap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Microphthalmia-associated Transcription Factor
Microphthalmia-associated transcription factor also known as class E basic helix-loop-helix protein 32 or bHLHe32 is a protein that in humans is encoded by the ''MITF'' gene. MITF is a basic helix-loop-helix leucine zipper transcription factor involved in lineage-specific pathway regulation of many types of cells including melanocytes, osteoclasts, and mast cells. The term "lineage-specific", since it relates to MITF, means genes or traits that are only found in a certain cell type. Therefore, MITF may be involved in the rewiring of signaling cascades that are specifically required for the survival and physiological function of their normal cell precursors. MITF, together with transcription factor EB ( TFEB), TFE3 and TFEC, belong to a subfamily of related bHLHZip proteins, termed the MiT-TFE family of transcription factors. The factors are able to form stable DNA-binding homo- and heterodimers. The gene that encodes for MITF resides at the ''mi'' locus in mice, and its pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Melanocyte
Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea), the inner ear, vaginal epithelium, meninges, bones, and heart. Melanin is a dark pigment primarily responsible for skin color. Once synthesized, melanin is contained in special organelles called melanosomes which can be transported to nearby keratinocytes to induce pigmentation. Thus darker skin tones have more melanosomes present than lighter skin tones. Functionally, melanin serves as protection against UV radiation. Melanocytes also have a role in the immune system. Function Through a process called melanogenesis, melanocytes produce melanin, which is a pigment found in the skin, eyes, hair, nasal cavity, and inner ear. This melanogenesis leads to a long-lasting pigmentation, which is in contrast to the pigmentation that originates from oxidation of already-existing melanin. There ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)


