|
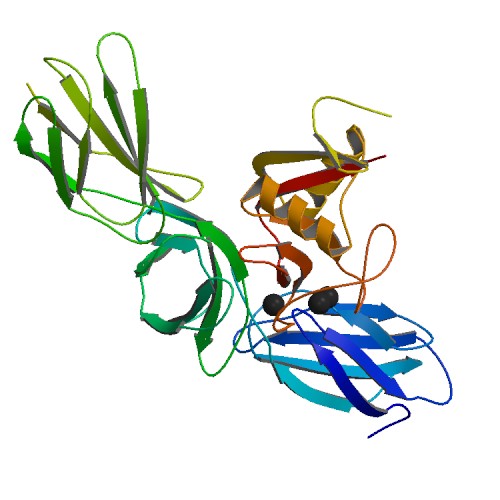
Aggrecanase
Aggrecanases are extracellular proteolytic enzymes that are members of the ADAMTS (A Disintegrin And Metalloprotease with Thrombospondin Motifs) family. Aggrecanases act on large proteoglycans known as aggrecans, which are components of connective tissues such as cartilage. The inappropriate activity of aggrecanase is a mechanism by which cartilage degradation occurs in diseases such as arthritis. At least two forms of aggrecanase exist in humans: ADAMTS4 or aggrecanase-1 and ADAMTS5 or aggrecanase-2. Both proteins contain thrombospondin Thrombospondins (TSPs) are a family of secreted glycoproteins with antiangiogenic functions. Due to their dynamic role within the extracellular matrix they are considered matricellular proteins. The first member of the family, thrombospondin 1 (T ... (TS) motifs required for proper recognition of substrates. Although both proteins can cleave the substrate aggrecan at the same position, they differ in kinetics and in secondary cleavage sites. Ref ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADAMTS4
A disintegrin and metalloproteinase with thrombospondin motifs 4 is an enzyme that in humans is encoded by the ''ADAMTS4'' gene. This gene encodes a member of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) protein family. Members of the family share several distinct protein modules, including a propeptide region, a metalloproteinase domain, a disintegrin-like domain, and a thrombospondin type 1 (TS) motif. Individual members of this family differ in the number of C-terminal TS motifs, and some have unique C-terminal domains. The enzyme encoded by this gene lacks a C-terminal TS motif. It can degrade aggrecan, a major proteoglycan of cartilage, brevican, a brain-specific extracellular matrix protein, neurocan and versican. The cleavage of aggrecan and brevican suggests key roles of this enzyme in arthritic disease and in the central nervous system, potentially, in the progression of glioma. Structure ADAMTS4 is the shortest known ADAMTS, lacking the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADAMTS5
A disintegrin and metalloproteinase with thrombospondin motifs 5 also known as ADAMTS5 is an enzyme that in humans is encoded by the ''ADAMTS5'' gene. Function ADAMTS5 is a member of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) protein family. Members of the family share several distinct protein modules, including a propeptide region, a metalloproteinase domain, a disintegrin-like domain, and a thrombospondin type 1 (TS) motif. Individual members of this family differ in the number of C-terminal TS motifs, and some have unique C-terminal domains. The enzyme encoded by this gene contains two C-terminal TS motifs and functions as aggrecanase to cleave aggrecan, a major proteoglycan of cartilage. ADAMTS5 may also have a role in the pathogenesis of human osteoarthritis. Animal studies Genetically modified mice in which the catalytic domain of ADAMTS5 was deleted are resistant to cartilage destruction in an experimental model of osteoarthritis. ADA ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteolytic
Proteolysis is the breakdown of proteins into smaller polypeptides or amino acids. Uncatalysed, the hydrolysis of peptide bonds is extremely slow, taking hundreds of years. Proteolysis is typically catalysed by cellular enzymes called proteases, but may also occur by intra-molecular digestion. Proteolysis in organisms serves many purposes; for example, digestive enzymes break down proteins in food to provide amino acids for the organism, while proteolytic processing of a polypeptide chain after its synthesis may be necessary for the production of an active protein. It is also important in the regulation of some physiological and cellular processes including apoptosis, as well as preventing the accumulation of unwanted or misfolded proteins in cells. Consequently, abnormality in the regulation of proteolysis can cause disease. Proteolysis can also be used as an analytical tool for studying proteins in the laboratory, and it may also be used in industry, for example in food process ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADAMTS
ADAMTS (short for a disintegrin and metalloproteinase with thrombospondin motifs) is a family of multidomain extracellular protease enzymes. 19 members of this family have been identified in humans, the first of which, ADAMTS1, was described in 1997. Known functions of the ADAMTS proteases include processing of procollagens and von Willebrand factor as well as cleavage of aggrecan, versican, brevican and neurocan, making them key remodeling enzymes of the extracellular matrix. They have been demonstrated to have important roles in connective tissue organization, coagulation, inflammation, arthritis, angiogenesis and cell migration. Homologous subfamily of ADAMTSL (ADAMTS-like) proteins, which lack enzymatic activity, has also been described. Most cases of thrombotic thrombocytopenic purpura arise from autoantibody-mediated inhibition of ADAMTS13. Like ADAMs, the name of the ADAMTS family refers to its disintegrin and metalloproteinase activity, and in the case of ADAMTS, the prese ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disintegrin
Disintegrins are a family of small proteins (45–84 amino acids in length) from viper venoms that function as potent inhibitors of both platelet aggregation and integrin-dependent cell adhesion. Operation Disintegrins work by countering the blood clotting steps, inhibiting the clumping of platelets. They interact with the beta-1 and -3 families of integrins receptors. Integrins are cell receptors involved in cell–cell and cell–extracellular matrix interactions, serving as the final common pathway leading to aggregation via formation of platelet–platelet bridges, which are essential in thrombosis and haemostasis. Disintegrins contain an RGD (Arg-Gly-Asp) or KGD (Lys-Gly-Asp) sequence motif that binds specifically to integrin IIb-IIIa receptors on the platelet surface, thereby blocking the binding of fibrinogen to the receptor–glycoprotein complex of activated platelets. Disintegrins act as receptor antagonists, inhibiting aggregation induced by ADP, thrombin, platelet-a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metalloprotease
A metalloproteinase, or metalloprotease, is any protease enzyme whose catalytic mechanism involves a metal. An example is ADAM12 which plays a significant role in the fusion of muscle cells during embryo development, in a process known as myogenesis. Most metalloproteases require zinc, but some use cobalt. The metal ion is coordinated to the protein via three ligands. The ligands coordinating the metal ion can vary with histidine, glutamate, aspartate, lysine, and arginine. The fourth coordination position is taken up by a labile water molecule. Treatment with chelating agents such as EDTA leads to complete inactivation. EDTA is a metal chelator that removes zinc, which is essential for activity. They are also inhibited by the chelator orthophenanthroline. Classification There are two subgroups of metalloproteinases: * Exopeptidases, metalloexopeptidases ( EC number: 3.4.17). * Endopeptidases, metalloendopeptidases (3.4.24). Well known metalloendopeptidases include ADAM prot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thrombospondin
Thrombospondins (TSPs) are a family of secreted glycoproteins with antiangiogenic functions. Due to their dynamic role within the extracellular matrix they are considered matricellular proteins. The first member of the family, thrombospondin 1 (THBS1), was discovered in 1971 by Nancy L. Baenziger. Types The thrombospondins are a family of multifunctional proteins. The family consists of thrombospondins 1-5 and can be divided into 2 subgroups: A, which contains TSP-1 and TSP-2, and B, which contains TSP-3, TSP-4 and TSP-5 (also designated cartilage oligomeric protein or COMP). TSP-1 and TSP-2 are homotrimers, consisting of three identical subunits, whereas TSP-3, TSP-4 and TSP-5 are homopentamers. TSP-1 and TSP-2 are produced by immature astrocytes during brain development, which promotes the development of new synapses. Thrombospondin 1 Thrombospondin 1 (TSP-1) is encoded by THBS1. It was first isolated from platelets that had been stimulated with thrombin, and so was design ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich proteoglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aggrecan
Aggrecan (ACAN), also known as cartilage-specific proteoglycan core protein (CSPCP) or chondroitin sulfate proteoglycan 1, is a protein that in humans is encoded by the ''ACAN'' gene. This gene is a member of the lectican (chondroitin sulfate proteoglycan) family. The encoded protein is an integral part of the extracellular matrix in cartilagenous tissue and it withstands compression in cartilage. Aggrecan is a proteoglycan, or a protein modified with large carbohydrates; the human form of the protein is 2316 amino acids long and can be expressed in multiple isoforms due to alternative splicing. Aggrecan was named for its ability to form large aggregates in the cartilage tissue (a large aggregating proteoglycan). Structure Aggrecan is a high molecular weight (1x106 < M < 3x106) proteoglycan. It exhibits a bottlebrush structure, in which |
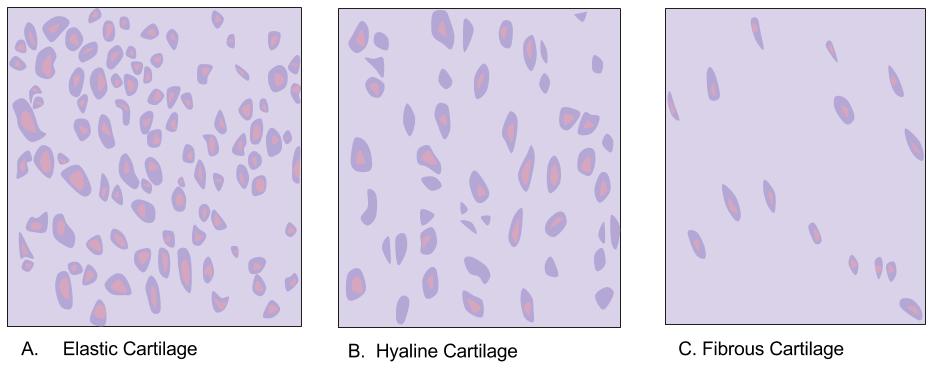
Cartilage
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. Because of its rigidity, cartilage often serves the purpose of holding tubes open in the body. Examples include the rings of the trachea, such as the cricoid cartilage and carina. Cartilage is composed of specialized cells called chondrocytes that produce a large amount of collagenous extracellular matrix, abundant ground substance that is rich in pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arthritis
Arthritis is a term often used to mean any disorder that affects joints. Symptoms generally include joint pain and stiffness. Other symptoms may include redness, warmth, swelling, and decreased range of motion of the affected joints. In some types of arthritis, other organs are also affected. Onset can be gradual or sudden. There are over 100 types of arthritis. The most common forms are osteoarthritis (degenerative joint disease) and rheumatoid arthritis. Osteoarthritis usually occurs with age and affects the fingers, knees, and hips. Rheumatoid arthritis is an autoimmune disorder that often affects the hands and feet. Other types include gout, lupus, fibromyalgia, and septic arthritis. They are all types of rheumatic disease. Treatment may include resting the joint and alternating between applying ice and heat. Weight loss and exercise may also be useful. Recommended medications may depend on the form of arthritis. These may include pain medications such as ibuprofen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |