|
Acoustic Wave
Acoustic waves are a type of energy propagation through a medium by means of adiabatic loading and unloading. Important quantities for describing acoustic waves are acoustic pressure, particle velocity, particle displacement and acoustic intensity. Acoustic waves travel with a characteristic acoustic velocity that depends on the medium they're passing through. Some examples of acoustic waves are audible sound from a speaker (waves traveling through air at the speed of sound), seismic waves (ground vibrations traveling through the earth), or ultrasound used for medical imaging (waves traveling through the body). Wave properties Acoustic wave equation The acoustic wave equation describes the propagation of sound waves. The acoustic wave equation for sound pressure in one dimension is given by - = 0 where *p is sound pressure in Pa *x is position in the direction of propagation of the wave, in m *c is speed of sound in m/s *t is time in s The wave equation for particl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acoustic Attenuation
Acoustic attenuation is a measure of the energy loss of sound propagation in media. Most media have viscosity and are therefore not ideal media. When sound propagates in such media, there is always thermal consumption of energy caused by viscosity. This effect can be quantified through the Stokes's law of sound attenuation. Sound attenuation may also be a result of heat conductivity in the media as has been shown by G. Kirchhoff in 1868. The Stokes-Kirchhoff attenuation formula takes into account both viscosity and thermal conductivity effects. For heterogeneous media, besides media viscosity, acoustic scattering is another main reason for removal of acoustic energy. Acoustic attenuation in a lossy medium plays an important role in many scientific researches and engineering fields, such as medical ultrasonography, vibration and noise reduction. Power-law frequency-dependent acoustic attenuation Many experimental and field measurements show that the acoustic attenuation coeffi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bulk Modulus
The bulk modulus (K or B) of a substance is a measure of how resistant to compression the substance is. It is defined as the ratio of the infinitesimal pressure increase to the resulting ''relative'' decrease of the volume. Other moduli describe the material's response (strain) to other kinds of stress: the shear modulus describes the response to shear stress, and Young's modulus describes the response to normal (lengthwise stretching) stress. For a fluid, only the bulk modulus is meaningful. For a complex anisotropic solid such as wood or paper, these three moduli do not contain enough information to describe its behaviour, and one must use the full generalized Hooke's law. The reciprocal of the bulk modulus at fixed temperature is called the isothermal compressibility. Definition The bulk modulus K (which is usually positive) can be formally defined by the equation :K=-V\frac , where P is pressure, V is the initial volume of the substance, and dP/dV denotes the derivative of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elastic Modulus
An elastic modulus (also known as modulus of elasticity) is the unit of measurement of an object's or substance's resistance to being deformed elastically (i.e., non-permanently) when a stress is applied to it. The elastic modulus of an object is defined as the slope of its stress–strain curve in the elastic deformation region: A stiffer material will have a higher elastic modulus. An elastic modulus has the form: :\delta \ \stackrel\ \frac where stress is the force causing the deformation divided by the area to which the force is applied and strain is the ratio of the change in some parameter caused by the deformation to the original value of the parameter. Since strain is a dimensionless quantity, the units of \delta will be the same as the units of stress. Specifying how stress and strain are to be measured, including directions, allows for many types of elastic moduli to be defined. The three primary ones are: # ''Young's modulus'' (E) describes tensile and compressive ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thermoacoustics
Thermoacoustics is the interaction between temperature, density and pressure variations of acoustic waves. Thermoacoustic heat engines can readily be driven using solar energy or waste heat Waste heat is heat that is produced by a machine, or other process that uses energy, as a byproduct of doing work. All such processes give off some waste heat as a fundamental result of the laws of thermodynamics. Waste heat has lower utility ... and they can be controlled using proportional control. They can use heat available at low temperatures which makes it ideal for heat recovery and low power applications. The components included in thermoacoustic engines are usually very simple compared to conventional Engine, engines. The device can easily be controlled and maintained. Thermoacoustic effects can be observed when partly molten glass tubes connected to glass vessels. Sometimes spontaneously a loud and monotone sound is produced. A similar effect is observed if one side of a stainle ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adiabatic Index
In thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or Laplace's coefficient, is the ratio of the heat capacity at constant pressure () to heat capacity at constant volume (). It is sometimes also known as the ''isentropic expansion factor'' and is denoted by ( gamma) for an ideal gasγ first appeared in an article by the French mathematician, engineer, and physicist Siméon Denis Poisson: * On p. 332, Poisson defines γ merely as a small deviation from equilibrium which causes small variations of the equilibrium value of the density ρ. In Poisson's article of 1823 – * γ was expressed as a function of density D (p. 8) or of pressure P (p. 9). Meanwhile, in 1816 the French mathematician and physicist Pierre-Simon Laplace had found that the speed of sound depends on the ratio of the specific heats. * However, he didn't denote the ratio as γ. In 1825, Laplace stated that the speed of sound is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Universal Gas Constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per amount of substance, i.e. the pressure–volume product, rather than energy per temperature increment per ''particle''. The constant is also a combination of the constants from Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. It is a physical constant that is featured in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is the constant of proportionality that relates the energy scale in physics to the temperature scale and the scale used for amount of substance. Thus, the value of the gas constant ultimately derives from historical decisions and accidents in the setting of units of energy, temperature and amount of substanc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mole (unit)
The mole, symbol mol, is the unit of amount of substance in the International System of Units (SI). The quantity amount of substance is a measure of how many elementary entities of a given substance are in an object or sample. The mole is defined as containing exactly elementary entities. Depending on what the substance is, an elementary entity may be an atom, a molecule, an ion, an ion pair, or a subatomic particle such as an electron. For example, 10 moles of water (a chemical compound) and 10 moles of mercury (a chemical element), contain equal amounts of substance and the mercury contains exactly one atom for each molecule of the water, despite the two having different volumes and different masses. The number of elementary entities in one mole is known as the Avogadro number, which is the approximate number of nucleons (protons or neutrons) in one gram of ordinary matter. The previous definition of a mole was simply the number of elementary entities equal to that of 12 gram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and even by industry. Further, both spellings are often used ''within'' a particular industry or country. Industries in British English-speaking countries typically use the "gauge" spelling. is the pressure relative to the ambient pressure. Various units are used to express pressure. Some of these derive from a unit of force divided by a unit of area; the SI unit of pressure, the pascal (Pa), for example, is one newton per square metre (N/m2); similarly, the pound-force per square inch (psi) is the traditional unit of pressure in the imperial and U.S. customary systems. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure, and the torr is defined as of this. Manometric u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ideal Gas Law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first stated by Benoît Paul Émile Clapeyron in 1834 as a combination of the empirical Boyle's law, Charles's law, Avogadro's law, and Gay-Lussac's law. The ideal gas law is often written in an empirical form: pV = nRT where p, V and T are the pressure, volume and temperature; n is the amount of substance; and R is the ideal gas constant. It can also be derived from the microscopic kinetic theory, as was achieved (apparently independently) by August Krönig in 1856 and Rudolf Clausius in 1857. Equation The state of an amount of gas is determined by its pressure, volume, and temperature. The modern form of the equation relates these simply in two main forms. The temperature used in the equation of state is an absolute temperature: the appropria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
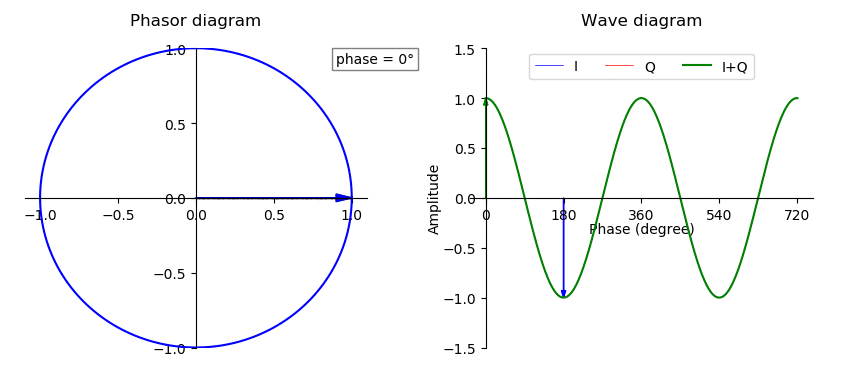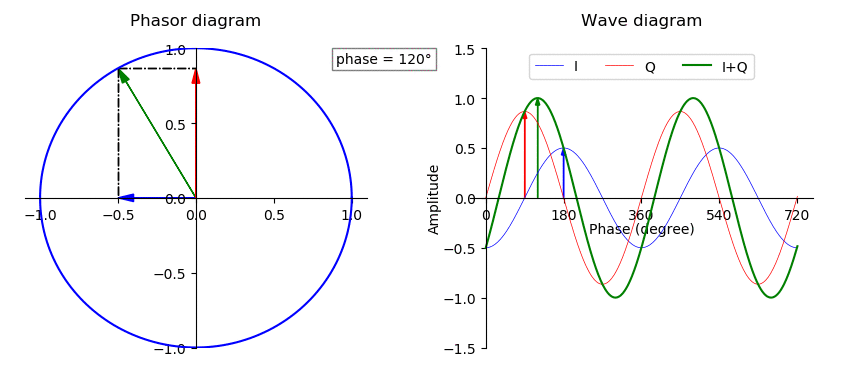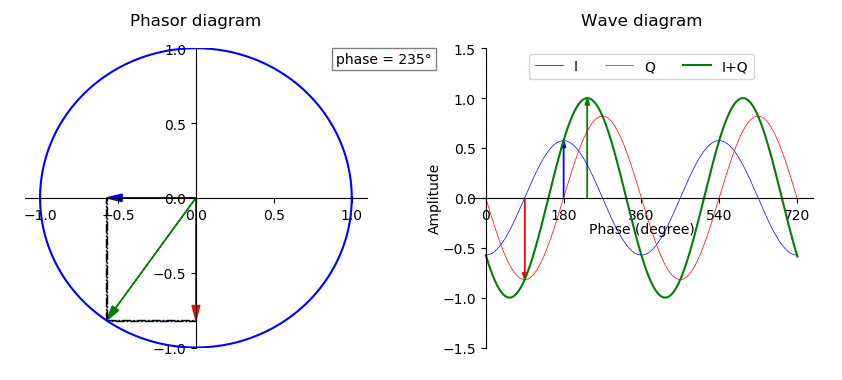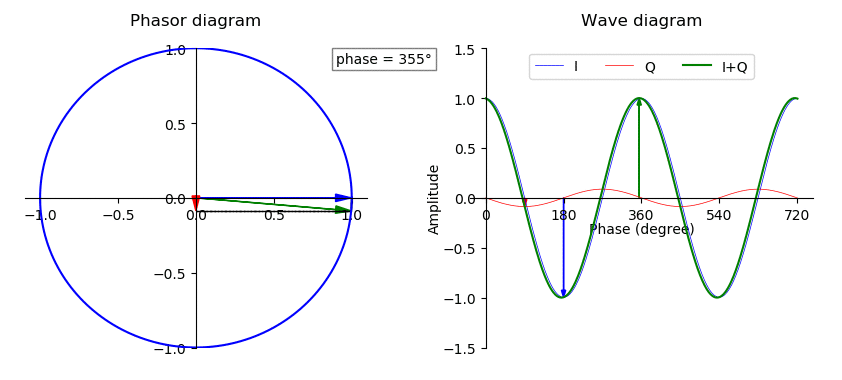
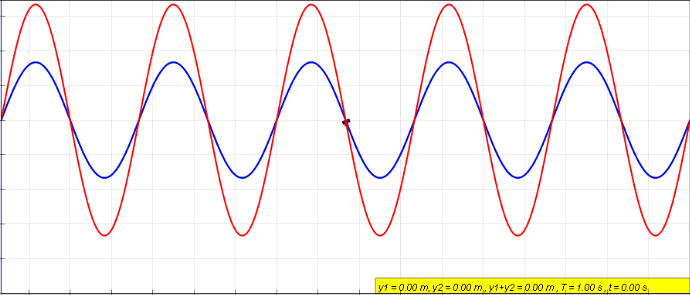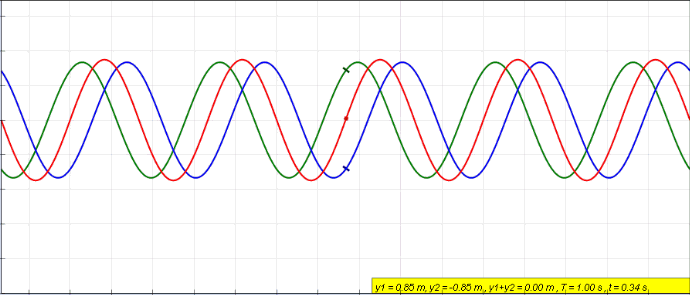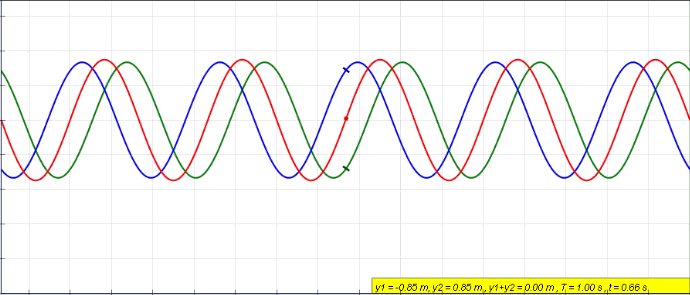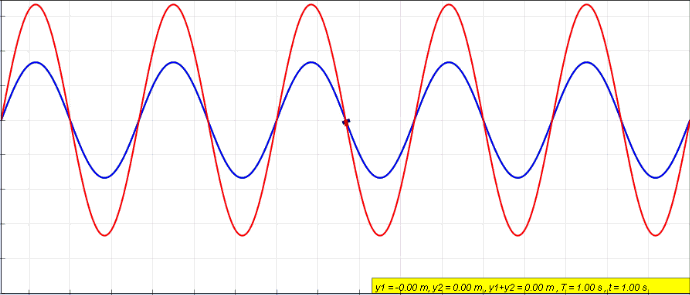
Phase (waves)
In physics and mathematics, the phase of a periodic function F of some real variable t (such as time) is an angle-like quantity representing the fraction of the cycle covered up to t. It is denoted \phi(t) and expressed in such a scale that it varies by one full turn as the variable t goes through each period (and F(t) goes through each complete cycle). It may be measured in any angular unit such as degrees or radians, thus increasing by 360° or 2\pi as the variable t completes a full period. This convention is especially appropriate for a sinusoidal function, since its value at any argument t then can be expressed as \phi(t), the sine of the phase, multiplied by some factor (the amplitude of the sinusoid). (The cosine may be used instead of sine, depending on where one considers each period to start.) Usually, whole turns are ignored when expressing the phase; so that \phi(t) is also a periodic function, with the same period as F, that repeatedly scans the same range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Standing Wave
In physics, a standing wave, also known as a stationary wave, is a wave that oscillates in time but whose peak amplitude profile does not move in space. The peak amplitude of the wave oscillations at any point in space is constant with respect to time, and the oscillations at different points throughout the wave are in phase. The locations at which the absolute value of the amplitude is minimum are called nodes, and the locations where the absolute value of the amplitude is maximum are called antinodes. Standing waves were first noticed by Michael Faraday in 1831. Faraday observed standing waves on the surface of a liquid in a vibrating container. Franz Melde coined the term "standing wave" (German: ''stehende Welle'' or ''Stehwelle'') around 1860 and demonstrated the phenomenon in his classic experiment with vibrating strings. This phenomenon can occur because the medium is moving in the direction opposite to the movement of the wave, or it can arise in a stationary medium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |