|
Aconitase
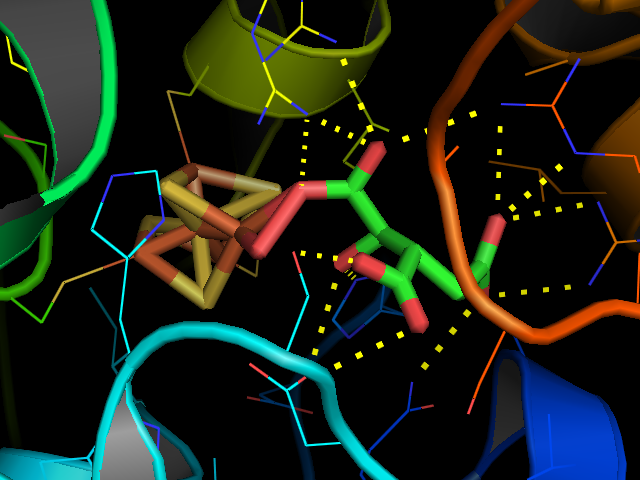
Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via ''cis''-aconitate in the tricarboxylic acid cycle, a non-redox-active process. Image:Citrate wpmp.png, Image:Cis-Aconitate wpmp.png, Image:isocitric acid.svg, Structure Aconitase, displayed in the structures in the right margin of this page, has two slightly different structures, depending on whether it is activated or inactivated. In the inactive form, its structure is divided into four domains. Counting from the N-terminus, only the first three of these domains are involved in close interactions with the Fe-4Scluster, but the active site consists of residues from all four domains, including the larger C-terminal domain. The Fe-S cluster and a anion also reside in the active site. When the enzyme is activated, it gains an additional iron atom, creating a Fe-4Scluster. However, the structure of the rest of the enzyme is nearly unchanged; the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-responsive Element-binding Protein
The iron-responsive element-binding proteins, also known as IRE-BP, IRBP, IRP and IFR , bind to iron-responsive elements (IREs) in the regulation of human iron metabolism. Function ACO1, or IRP1, is a bifunctional protein that functions as an iron-responsive element (IRE)-binding protein involved in the control of iron metabolism by binding mRNA to repress translation or degradation. It functions also as the cytoplasmic isoform of aconitase. Aconitases are iron-sulfur proteins that require a 4Fe-4S cluster for their enzymatic activity, in which they catalyze conversion of citrate to isocitrate. This structure was based on x-ray crystal diffraction. The resolution was 2.80 Å. This protein was harvested from the species '' Oryctolagus cuniculus'', more commonly known as a rabbit. This protein has a couple of conformational changes associated with it to explain the alternative functions as either mRNA regulator or as an enzyme. This information was obtained from the RCSB protein ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iron-sulfur Cluster
Iron–sulfur proteins (or iron–sulphur proteins in British spelling) are proteins characterized by the presence of iron–sulfur clusters containing sulfide-linked di-, tri-, and tetrairon centers in variable oxidation states. Iron–sulfur clusters are found in a variety of metalloproteins, such as the ferredoxins, as well as NADH dehydrogenase, hydrogenases, coenzyme Q – cytochrome c reductase, succinate – coenzyme Q reductase and nitrogenase. Iron–sulfur clusters are best known for their role in the oxidation-reduction reactions of electron transport in mitochondria and chloroplasts. Both Complex I and Complex II of oxidative phosphorylation have multiple Fe–S clusters. They have many other functions including catalysis as illustrated by aconitase, generation of radicals as illustrated by SAM-dependent enzymes, and as sulfur donors in the biosynthesis of lipoic acid and biotin. Additionally, some Fe–S proteins regulate gene expression. Fe–S proteins ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47&nbs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocitrate
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salts and esters of isocitric acid are known as isocitrates. The isocitrate anion is a substrate of the citric acid cycle. Isocitrate is formed from citrate with the help of the enzyme aconitase Aconitase (aconitate hydratase; ) is an enzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via ''cis''- aconitate in the tricarboxylic acid cycle, a non-redox-active process. Image:Citrate wpmp.png, Image:Cis- ..., and is acted upon by isocitrate dehydrogenase. Isocitric acid is commonly used as a marker to detect the authenticity and quality of fruit products, most often citrus juices. In authentic orange juice, for example, the ratio of citric acid to D-isocitric acid is usually less than 130. An isocitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aconitate
Aconitic acid is an organic acid. The two isomers are ''cis''-aconitic acid and ''trans''-aconitic acid. The conjugate base of ''cis''-aconitic acid, ''cis''-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase. Aconitic acid can be synthesized by dehydration of citric acid Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ... using sulfuric acid: :(HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O A mixture of isomers are generated in this way. It was first prepared by thermal dehydration. References {{Citric acid cycle Tricarboxylic acids Enoic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Isopropylmalate Dehydratase
3-Isopropylmalate dehydratase () is an aconitase homologue, which catalyses the isomerisation of 2-isopropylmalate to 3-isopropylmalate, via dehydration, in the biosynthesis of leucine Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- .... References External links * {{enzyme-stub Lyases ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citric Acid
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aconitic Acid
Aconitic acid is an organic acid. The two isomers are ''cis''-aconitic acid and ''trans''-aconitic acid. The conjugate base of ''cis''-aconitic acid, ''cis''-aconitate is an intermediate in the isomerization of citrate to isocitrate in the citric acid cycle. It is acted upon by the enzyme aconitase. Aconitic acid can be synthesized by dehydration of citric acid Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in t ... using sulfuric acid: :(HO2CCH2)2C(OH)CO2H → HO2CCH=C(CO2H)CH2CO2H + H2O A mixture of isomers are generated in this way. It was first prepared by thermal dehydration. References {{Citric acid cycle Tricarboxylic acids Enoic acids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isocitric Acid
Isocitric acid is a structural isomer of citric acid. Since citric acid and isocitric acid are structural isomers, they share similar physical and chemical properties. Due to these similar properties, it is difficult to separate the isomers. Salts and esters of isocitric acid are known as isocitrates. The isocitrate anion is a substrate of the citric acid cycle. Isocitrate is formed from citrate with the help of the enzyme aconitase, and is acted upon by isocitrate dehydrogenase. Isocitric acid is commonly used as a marker to detect the authenticity and quality of fruit products, most often citrus juices. In authentic orange juice, for example, the ratio of citric acid to D-isocitric acid is usually less than 130. An isocitric acid value higher than this may be indicative of fruit juice adulteration. Isocitric acid has largely been used as a biochemical agent due to limited amounts. However, isocitric acid has been shown to have pharmaceutical and therapeutic effects. Isocitric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Citrate Zoom Final
Citric acid is an organic compound with the chemical formula HOC(CO2H)(CH2CO2H)2. It is a colorless weak organic acid. It occurs naturally in citrus fruits. In biochemistry, it is an intermediate in the citric acid cycle, which occurs in the metabolism of all aerobic organisms. More than two million tons of citric acid are manufactured every year. It is used widely as an acidifier, as a flavoring, and a chelating agent. A citrate is a derivative of citric acid; that is, the salts, esters, and the polyatomic anion found in solution. An example of the former, a salt is trisodium citrate; an ester is triethyl citrate. When part of a salt, the formula of the citrate anion is written as or . Natural occurrence and industrial production Citric acid occurs in a variety of fruits and vegetables, most notably citrus fruits. Lemons and limes have particularly high concentrations of the acid; it can constitute as much as 8% of the dry weight of these fruits (about 47 g/L i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question (dynamic stereochem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |