|
Acid–base Disorder
Acid–base imbalance is an abnormality of the human body's normal balance of acids and bases that causes the plasma pH to deviate out of the normal range (7.35 to 7.45). In the fetus, the normal range differs based on which umbilical vessel is sampled (umbilical vein pH is normally 7.25 to 7.45; umbilical artery pH is normally 7.18 to 7.38). It can exist in varying levels of severity, some life-threatening. Classification An excess of acid is called acidosis or acidemia, while an excess in bases is called alkalosis or alkalemia. The process that causes the imbalance is classified based on the cause of the disturbance (respiratory or metabolic) and the direction of change in pH (acidosis or alkalosis). This yields the following four basic processes: Mixed disorders The presence of only one of the above derangements is called a ''simple'' acid–base disorder. In a ''mixed'' disorder, more than one is occurring at the same time. Mixed disorders may feature an acidosis and alko ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Davenport Diagram
In acid base physiology, the Davenport diagram is a graphical tool, developed by Horace W. Davenport, that allows a clinician or investigator to describe blood bicarbonate concentrations and blood pH following a respiratory and/or metabolic acid-base disturbance. The diagram depicts a three-dimensional surface describing all possible states of chemical equilibria between gaseous carbon dioxide, aqueous bicarbonate and aqueous protons at the physiologically complex interface of the Pulmonary alveolus, alveoli of the lungs and the alveolar capillaries. Although the surface represented in the diagram is experimentally determined, the Davenport diagram is rarely used in the clinical setting, but allows the investigator to envision the effects of physiological changes on blood acid-base chemistry. For clinical use there are two recent innovations: an Acid-Base Diagram which provides Text Descriptions for the abnormalities and a High Altitude Version that provides text descriptions appropr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter A Stewart
Peter Arthur Robert Stewart (1921–1993) was a Canadian physiologist who introduced an alternate approach to understanding acid–base physiology. He outlined his model in a paper in 1978, and explained it his 1981 book, ''How to Understand Acid–Base''. The book was unavailable for many years, then made available on-line and finally reprinted in 2009, with additional chapters on current applications in clinical medicine. The Stewart approach models the complex chemical equilibrium system known as acid–base balance. Stewart introduced the term "strong ion difference" or IDto mean the concentration of strongly dissociating cations minus the concentration of strongly dissociating anions. He characterised this, the total weak acid concentration and the partial pressure of CO2 as independent variables and formulated a quartic equation relating +to these three independent variables. The quartic equation was solved numerically by computer and has never been validated by titrati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia
Ammonia is an inorganic compound of nitrogen and hydrogen with the formula . A stable binary hydride, and the simplest pnictogen hydride, ammonia is a colourless gas with a distinct pungent smell. Biologically, it is a common nitrogenous waste, particularly among aquatic organisms, and it contributes significantly to the nutritional needs of terrestrial organisms by serving as a precursor to 45% of the world's food and fertilizers. Around 70% of ammonia is used to make fertilisers in various forms and composition, such as urea and Diammonium phosphate. Ammonia in pure form is also applied directly into the soil. Ammonia, either directly or indirectly, is also a building block for the synthesis of many pharmaceutical products and is used in many commercial cleaning products. It is mainly collected by downward displacement of both air and water. Although common in nature—both terrestrially and in the outer planets of the Solar System—and in wide use, ammonia is both caust ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Extracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions from sub-disciplines and related fields, see Glossary of genetics, Glossary of evolutionary biology, Glossary of ecology, and Glossary of scientific naming, or any of the organism-specific glossaries in :Glossaries of biology. A B C D E ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffering Agent
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen ion ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperventilation
Hyperventilation is irregular breathing that occurs when the rate or tidal volume of breathing eliminates more carbon dioxide than the body can produce. This leads to hypocapnia, a reduced concentration of carbon dioxide dissolved in the blood. The body normally attempts to compensate for this homeostatically, but if this fails or is overridden, the blood pH will rise, leading to respiratory alkalosis. The symptoms of respiratory alkalosis include: dizziness, tingling in the lips, hands or feet, headache, weakness, fainting, and seizures. In extreme cases it may cause carpopedal spasms, a flapping and contraction of the hands and feet. Factors that may induce or sustain hyperventilation include: physiological stress, anxiety or panic disorder, high altitude, head injury, stroke, respiratory disorders such as asthma, pneumonia, or hyperventilation syndrome, cardiovascular problems such as pulmonary embolisms, anemia, an incorrectly calibrated medical respirator, and adverse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diarrhea
Diarrhea, also spelled diarrhoea, is the condition of having at least three loose, liquid, or watery bowel movements each day. It often lasts for a few days and can result in dehydration due to fluid loss. Signs of dehydration often begin with loss of the normal stretchiness of the skin and irritable behaviour. This can progress to decreased urination, loss of skin color, a fast heart rate, and a decrease in responsiveness as it becomes more severe. Loose but non-watery stools in babies who are exclusively breastfed, however, are normal. The most common cause is an infection of the intestines due to either a virus, bacterium, or parasite—a condition also known as gastroenteritis. These infections are often acquired from food or water that has been contaminated by feces, or directly from another person who is infected. The three types of diarrhea are: short duration watery diarrhea, short duration bloody diarrhea, and persistent diarrhea (lasting more than two weeks, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
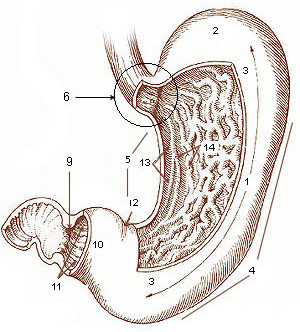
Gastric Aspiration
The stomach is a muscular, hollow organ in the gastrointestinal tract of humans and many other animals, including several invertebrates. The stomach has a dilated structure and functions as a vital organ in the digestive system. The stomach is involved in the gastric phase of digestion, following chewing. It performs a chemical breakdown by means of enzymes and hydrochloric acid. In humans and many other animals, the stomach is located between the oesophagus and the small intestine. The stomach secretes digestive enzymes and gastric acid to aid in food digestion. The pyloric sphincter controls the passage of partially digested food (chyme) from the stomach into the duodenum, where peristalsis takes over to move this through the rest of intestines. Structure In the human digestive system, the stomach lies between the oesophagus and the duodenum (the first part of the small intestine). It is in the left upper quadrant of the abdominal cavity. The top of the stomach lies against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vomitus
Vomiting (also known as emesis and throwing up) is the involuntary, forceful expulsion of the contents of one's stomach through the mouth and sometimes the nose. Vomiting can be the result of ailments like food poisoning, gastroenteritis, pregnancy, motion sickness, or hangover; or it can be an after effect of diseases such as brain tumors, elevated intracranial pressure, or overexposure to ionizing radiation. The feeling that one is about to vomit is called nausea; it often precedes, but does not always lead to vomiting. Impairment due to alcohol or anesthesia can cause inhalation of vomit, leading to suffocation. In severe cases, where dehydration develops, intravenous fluid may be required. Antiemetics are sometimes necessary to suppress nausea and vomiting. Self-induced vomiting can be a component of an eating disorder such as bulimia, and is itself now classified as an eating disorder on its own, purging disorder. Complications Aspiration Vomiting is dangerous if g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Urine
Urine is a liquid by-product of metabolism in humans and in many other animals. Urine flows from the kidneys through the ureters to the urinary bladder. Urination results in urine being excretion, excreted from the body through the urethra. Cell (biology), Cellular metabolism generates many by-products that are rich in nitrogen and must be clearance (medicine), cleared from the Circulatory system, bloodstream, such as urea, uric acid, and creatinine. These by-products are expelled from the body during urination, which is the primary method for excreting water-soluble chemicals from the body. A urinalysis can detect nitrogenous wastes of the mammalian body. Urine plays an important role in the earth's nitrogen cycle. In balanced ecosystems, urine fertilizes the soil and thus helps plants to grow. Therefore, Reuse of excreta, urine can be used as a fertilizer. Some animals use it to territory (animal)#Scent marking, mark their territories. Historically, aged or fermented urine (kn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Feces
Feces ( or faeces), known colloquially and in slang as poo and poop, are the solid or semi-solid remains of food that was not digested in the small intestine, and has been broken down by bacteria in the large intestine. Feces contain a relatively small amount of metabolic waste products such as bacterially altered bilirubin, and dead epithelial cells from the lining of the gut. Feces are discharged through the anus or cloaca during defecation. Feces can be used as fertilizer or soil conditioner in agriculture. They can also be burned as fuel or dried and used for construction. Some medicinal uses have been found. In the case of human feces, fecal transplants or fecal bacteriotherapy are in use. Urine and feces together are called excreta. Skatole is the principal compound responsible for the unpleasant smell of feces. Characteristics The distinctive odor of feces is due to skatole, and thiols (sulfur-containing compounds), as well as amines and carboxylic acids. Skatole ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


-3D-balls.png)