|
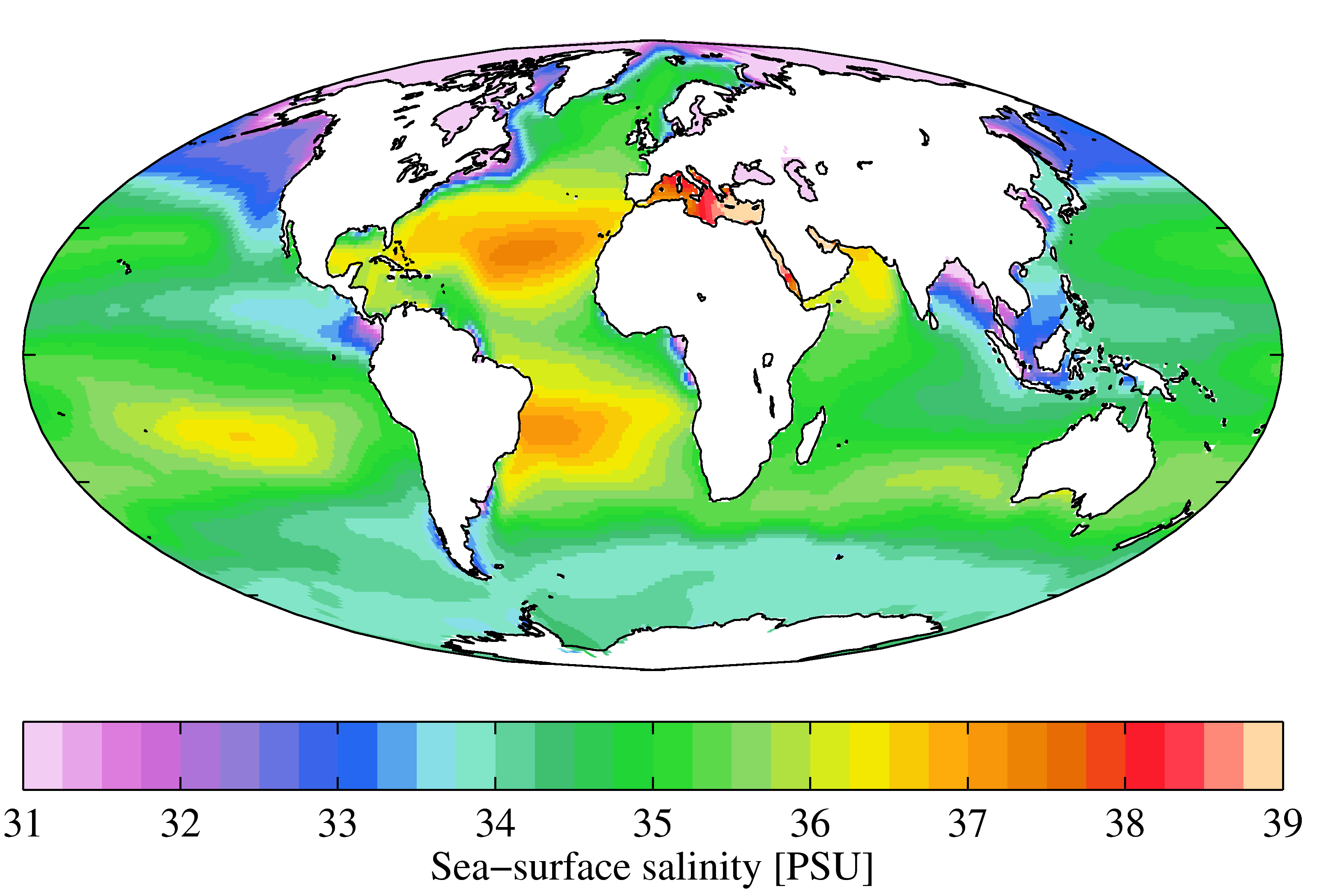
Seawater
Seawater, or sea water, is water from a sea or ocean. On average, seawater in the world's oceans has a salinity of about 3.5% (35 g/L, 35 ppt, 600 mM). This means that every kilogram (roughly one liter by volume) of seawater has approximately of dissolved salts (predominantly sodium () and chloride () ions). The average density at the surface is 1.025 kg/L. Seawater is denser than both fresh water and pure water (density 1.0 kg/L at ) because the dissolved salts increase the mass by a larger proportion than the volume. The freezing point of seawater decreases as salt concentration increases. At typical salinity, it freezes at about . The coldest seawater still in the liquid state ever recorded was found in 2010, in a stream under an Antarctic glacier: the measured temperature was . Seawater pH is typically limited to a range between 7.5 and 8.4. However, there is no universally accepted reference pH-scale for seawater and the difference between measurements bas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
San Andrés (island)
, native_name_lang = icr , sobriquet = , image_name = San Andres Island Montage.jpg , image_size = 300px , image_caption = Top to bottom, left to right: A typical San Andrés house, San Andrés Skyline, Johnny Cay, Spratt Bight Beach, First Baptist Church and the Sunrise Hotel , image_alt = , map_image = San Andres Island.png , map_caption = , location = , pushpin_map = Colombia Isla de San Andrés#Colombia#Caribbean , pushpin_label = , pushpin_label_position = left , pushpin_map_alt = , pushpin_relief = 1 , pushpin_map_caption = , coordinates = , archipelago = , total_islands = , major_islands = San Andrés, Providence and Saint Catherine , are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitation (meteorology)
In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls under gravitational pull from clouds. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor (reaching 100% relative humidity), so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation but colloids, because the water vapor does not condense sufficiently to precipitate. Two processes, possibly acting together, can lead to air becoming saturated: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are called showers. Moisture that is lifted or otherwise forced to rise over a layer of sub-freezing air at the surface may be condense ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium
Calcium is a chemical element with the symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to its heavier homologues strontium and barium. It is the fifth most abundant element in Earth's crust, and the third most abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early sea life; gypsum, anhydrite, fluorite, and apatite are also sources of calcium. The name derives from Latin ''calx'' "lime", which was obtained from heating limestone. Some calcium compounds were known to the ancients, though their chemistry was unknown until the seventeenth century. Pure calcium was isolated in 1808 via electrolysis of its oxide by Humphry Davy, who named the element. Calcium compounds are widely used in many industries: in foods and pharma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorine
Chlorine is a chemical element with the Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between them. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity on the revised Electronegativity#Pauling electronegativity, Pauling scale, behind only oxygen and fluorine. Chlorine played an important role in the experiments conducted by medieval Alchemy, alchemists, which commonly involved the heating of chloride Salt (chemistry), salts like ammonium chloride (sal ammoniac) and sodium chloride (common salt), producing various chemical substances containing chlorine such as hydrogen chloride, mercury(II) chloride (corrosive sublimate), and hydrochloric acid (in the form of ). However ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residence Time (fluid Dynamics)
The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume (e.g.: a chemical reactor, a lake, a human body). The residence time of a set of parcels is quantified in terms of the frequency distribution of the residence time in the set, which is known as residence time distribution (RTD), or in terms of its average, known as mean residence time. Residence time plays an important role in chemistry and especially in environmental science and pharmacology. Under the name ''lead time'' or ''waiting time'' it plays a central role respectively in supply chain management and queueing theory, where the material that flows is usually discrete instead of continuous. History The concept of residence time originated in models of chemical reactors. The first such model was an ''axial dispersion model'' by Irving Langmuir in 1908. This received little attention for 45 years; other models were developed such as the plug flow reactor model and the con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Percentage
In mathematics, a percentage (from la, per centum, "by a hundred") is a number or ratio expressed as a fraction of 100. It is often denoted using the percent sign, "%", although the abbreviations "pct.", "pct" and sometimes "pc" are also used. A percentage is a dimensionless number (pure number); it has no unit of measurement. Examples For example, 45% (read as "forty-five per cent") is equal to the fraction , the ratio 45:55 (or 45:100 when comparing to the total rather than the other portion), or 0.45. Percentages are often used to express a proportionate part of a total. (Similarly, one can also express a number as a fraction of 1,000, using the term "per mille" or the symbol "".) Example 1 If 50% of the total number of students in the class are male, that means that 50 out of every 100 students are male. If there are 500 students, then 250 of them are male. Example 2 An increase of $0.15 on a price of $2.50 is an increase by a fraction of = 0.06. Expressed as a p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid . The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid ; and the conjugate acid of , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Oceanography
Marine chemistry, also known as ocean chemistry or chemical oceanography, is influenced by plate tectonics and seafloor spreading, turbidity currents, sediments, pH levels, atmospheric constituents, metamorphic activity, and ecology. The field of chemical oceanography studies the chemistry of marine environments including the influences of different variables. Marine life has adapted to the chemistries unique to earth's oceans, and marine ecosystems are sensitive to changes in ocean chemistry. The impact of human activity on the chemistry of the earth's oceans has increased over time, with pollution from industry and various land-use practices significantly affecting the oceans. Moreover, increasing levels of carbon dioxide in the earth's atmosphere have led to ocean acidification, which has negative effects on marine ecosystems. The international community has agreed that restoring the chemistry of the oceans is a priority, and efforts toward this goal are tracked as part of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Property
A chemical property is any of a material's properties that becomes evident during, or after, a chemical reaction; that is, any quality that can be established only by changing a substance's chemical identity.William L. Masterton, Cecile N. Hurley, "Chemistry: Principles and Reactions", 6th edition. Brooks/Cole Cengage Learning, 2009, p.1(Google books)/ref> Simply speaking, chemical properties cannot be determined just by viewing or touching the substance; the substance's internal structure must be affected greatly for its chemical properties to be investigated. When a substance goes under a chemical reaction, the properties will change drastically, resulting in chemical change. However, a catalytic property would also be a chemical property. Chemical properties can be contrasted with physical properties, which can be discerned without changing the substance's structure. However, for many properties within the scope of physical chemistry, and other disciplines at the boundary bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biological Productivity
In ecology, the term productivity refers to the rate of generation of Biomass (ecology), biomass in an ecosystem, usually expressed in units of mass per volume (unit surface) per unit of time, such as grams per square metre per day (g m−2 d−1). The unit of mass can relate to dry matter or to the mass of generated carbon-based life, carbon. The productivity of autotrophs, such as plants, is called primary productivity, while the productivity of heterotrophs, such as animals, is called secondary productivity. Primary production Primary production is the synthesis of Organic compound, organic material from Inorganic compound, inorganic molecules. Primary production in most ecosystems is dominated by the process of photosynthesis, In which organisms synthesize organic molecules from sunlight, H2O, H2O, and CO2, CO2. Primary production is sometimes broken down into Net Primary Production (NPP) and Gross Primary Production (GPP). Gross primary production measures all carbon assimil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide Emissions
Greenhouse gas emissions from human activities strengthen the greenhouse effect, contributing to climate change. Most is carbon dioxide from burning fossil fuels: coal, oil, and natural gas. The largest emitters include coal in China and large oil and gas companies, many state-owned by OPEC and Russia. Human-caused emissions have increased atmospheric carbon dioxide by about 50% over pre-industrial levels. The growing levels of emissions have varied, but it was consistent among all greenhouse gases (GHG). Emissions in the 2010s averaged 56 billion tons a year, higher than ever before. Electricity generation and transport are major emitters; the largest single source, according to the United States Environmental Protection Agency, is transportation, accounting for 27% of all USA greenhouse gas emissions. Deforestation and other changes in land use also emit carbon dioxide and methane. The largest source of anthropogenic methane emissions is agriculture, closely followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
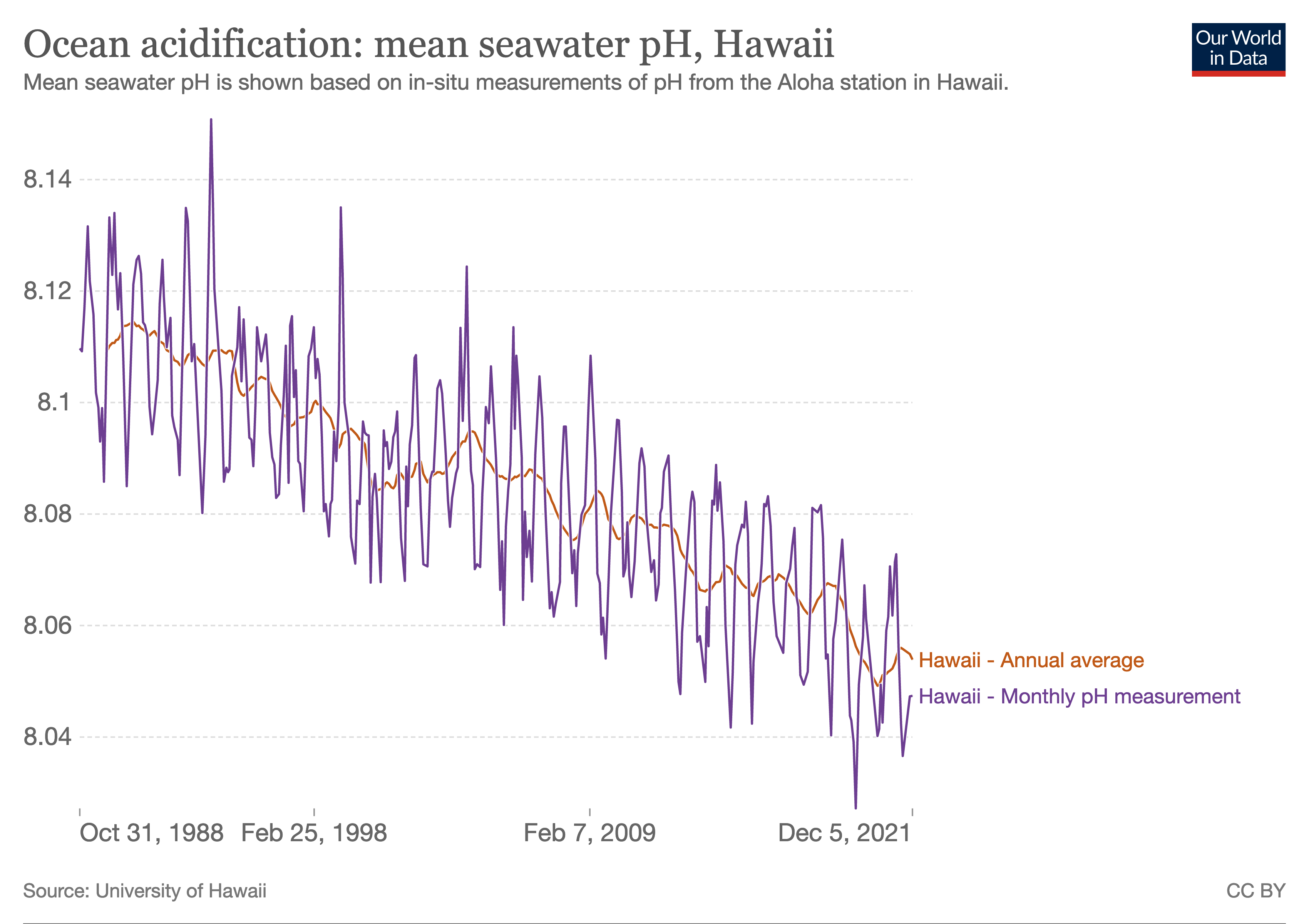
Ocean Acidification
Ocean acidification is the reduction in the pH value of the Earth’s ocean. Between 1751 and 2021, the average pH value of the ocean surface has decreased from approximately 8.25 to 8.14. The root cause of ocean acidification is carbon dioxide emissions from human activities which have led to atmospheric carbon dioxide (CO2) levels of more than 410 ppm (in 2020). The oceans absorb CO2 from the atmosphere. This leads to the formation of carbonic acid (H2CO3) which dissociates into a bicarbonate ion () and a hydrogen ion (H+). The free hydrogen ions (H+) decrease the pH of the ocean, therefore increasing acidity (this does not mean that seawater is acidic yet; it is still alkaline, with a pH higher than 8). A decrease in pH corresponds to a decrease in the concentration of carbonate ions, which are the main building block for calcium carbonate (CaCO3) shells and skeletons. Marine calcifying organisms, like mollusks, oysters and corals, are particularly affected by this as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |