|
Cal BP
Before Present (BP) years, or "years before present", is a time scale used mainly in archaeology, geology and other scientific disciplines to specify when events occurred relative to the origin of practical radiocarbon dating in the 1950s. Because the "present" time changes, standard practice is to use 1 January 1950 as the commencement date (epoch) of the age scale. The abbreviation "BP" has been interpreted retrospectively as "Before Physics", which refers to the time before nuclear weapons testing artificially altered the proportion of the carbon isotopes in the atmosphere, which scientists must now account for. In a convention that is not always observed, many sources restrict the use of BP dates to those produced with radiocarbon dating; the alternative notation RCYBP stands for the explicit "radio carbon years before present". Usage The BP scale is sometimes used for dates established by means other than radiocarbon dating, such as stratigraphy. This usage differs from ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Geologic Time Scale
The geologic time scale, or geological time scale, (GTS) is a representation of time based on the rock record of Earth. It is a system of chronological dating that uses chronostratigraphy (the process of relating strata to time) and geochronology (scientific branch of geology that aims to determine the age of rocks). It is used primarily by Earth scientists (including geologists, paleontologists, geophysicists, geochemists, and paleoclimatologists) to describe the timing and relationships of events in geologic history. The time scale has been developed through the study of rock layers and the observation of their relationships and identifying features such as lithologies, paleomagnetic properties, and fossils. The definition of standardized international units of geologic time is the responsibility of the International Commission on Stratigraphy (ICS), a constituent body of the International Union of Geological Sciences (IUGS), whose primary objective is to precisely define ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiocarbon Dating
Radiocarbon dating (also referred to as carbon dating or carbon-14 dating) is a method for determining the age of an object containing organic material by using the properties of radiocarbon, a radioactive isotope of carbon. The method was developed in the late 1940s at the University of Chicago by Willard Libby. It is based on the fact that radiocarbon () is constantly being created in the Earth's atmosphere by the interaction of cosmic rays with atmospheric nitrogen. The resulting combines with atmospheric oxygen to form radioactive carbon dioxide, which is incorporated into plants by photosynthesis; animals then acquire by eating the plants. When the animal or plant dies, it stops exchanging carbon with its environment, and thereafter the amount of it contains begins to decrease as the undergoes radioactive decay. Measuring the amount of in a sample from a dead plant or animal, such as a piece of wood or a fragment of bone, provides information that can be used to calc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
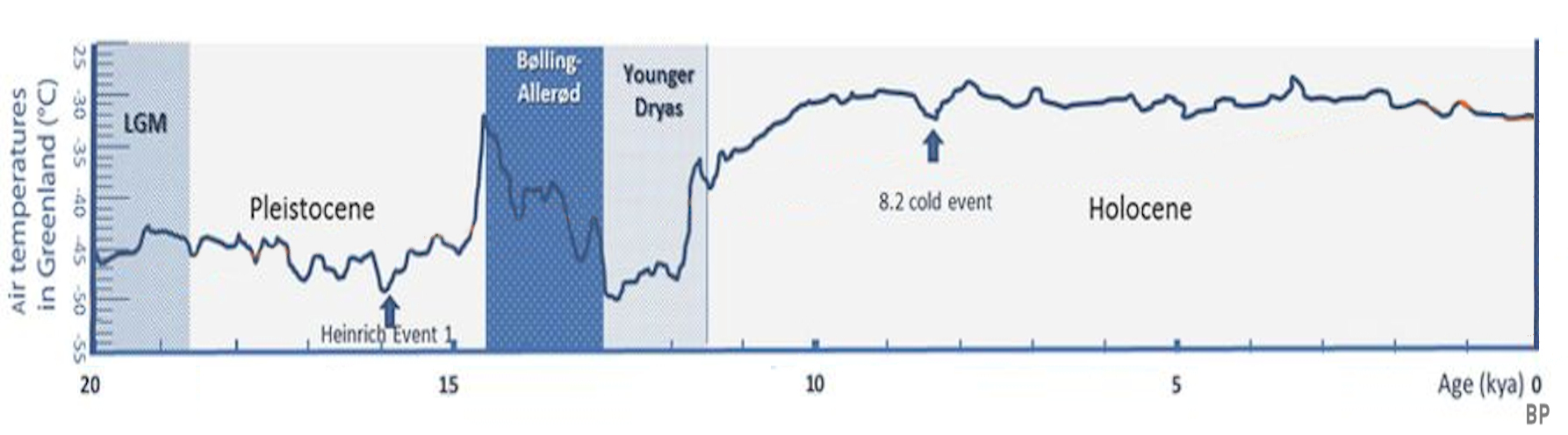
Pleistocene
The Pleistocene ( , often referred to as the ''Ice age'') is the geological Epoch (geology), epoch that lasted from about 2,580,000 to 11,700 years ago, spanning the Earth's most recent period of repeated glaciations. Before a change was finally confirmed in 2009 by the International Union of Geological Sciences, the cutoff of the Pleistocene and the preceding Pliocene was regarded as being 1.806 million years Before Present (BP). Publications from earlier years may use either definition of the period. The end of the Pleistocene corresponds with the end of the last glacial period and also with the end of the Paleolithic age used in archaeology. The name is a combination of Ancient Greek grc, label=none, πλεῖστος, pleīstos, most and grc, label=none, καινός, kainós (latinized as ), 'new'. At the end of the preceding Pliocene, the previously isolated North and South American continents were joined by the Isthmus of Panama, causing Great American Interchang ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
10th Millennium BC
The 10th millennium BC spanned the years 10,000 BC to 9001 BC (c. 12 ka to c. 11 ka). It marks the beginning of the transition from the Palaeolithic to the Neolithic via the interim Mesolithic ( Northern Europe and Western Europe) and Epipaleolithic (Levant and Near East) periods, which together form the first part of the Holocene epoch that is generally believed to have begun c. 9700 BC (c. 11.7 ka) and is the current geological epoch. It is impossible to precisely date events that happened around the time of this millennium, and all dates mentioned here are estimates mostly based on geological, anthropological analysis, and radiometric dating. Holocene epoch The main characteristic of the Holocene has been the worldwide abundance of ''Homo sapiens sapiens'' (humankind). The epoch began in the wake of the Würm glaciation, generally known as the Last Ice Age, which began 109 ka and ended 14 ka when ''Homo sapiens sapiens'' was in the Palaeolithic (Old Stone) Age. Followi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gregorian Calendar
The Gregorian calendar is the calendar used in most parts of the world. It was introduced in October 1582 by Pope Gregory XIII as a modification of, and replacement for, the Julian calendar. The principal change was to space leap years differently so as to make the average calendar year 365.2425 days long, more closely approximating the 365.2422-day 'tropical' or 'solar' year that is determined by the Earth's revolution around the Sun. The rule for leap years is: There were two reasons to establish the Gregorian calendar. First, the Julian calendar assumed incorrectly that the average solar year is exactly 365.25 days long, an overestimate of a little under one day per century, and thus has a leap year every four years without exception. The Gregorian reform shortened the average (calendar) year by 0.0075 days to stop the drift of the calendar with respect to the equinoxes.See Wikisource English translation of the (Latin) 1582 papal bull '' Inter gravissimas''. Second, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dendrochronology
Dendrochronology (or tree-ring dating) is the scientific method of dating tree rings (also called growth rings) to the exact year they were formed. As well as dating them, this can give data for dendroclimatology, the study of climate and atmospheric conditions during different periods in history from wood. Dendrochronology derives from Ancient Greek (), meaning "tree", (), meaning "time", and (), "the study of". Dendrochronology is useful for determining the precise age of samples, especially those that are too recent for radiocarbon dating, which always produces a range rather than an exact date. However, for a precise date of the death of the tree a full sample to the edge is needed, which most trimmed timber will not provide. It also gives data on the timing of events and rates of change in the environment (most prominently climate) and also in wood found in archaeology or works of art and architecture, such as old panel paintings. It is also used as a check in radiocar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-12
Carbon-12 (12C) is the most abundant of the two stable isotopes of carbon (carbon-13 being the other), amounting to 98.93% of element carbon on Earth; its abundance is due to the triple-alpha process by which it is created in stars. Carbon-12 is of particular importance in its use as the standard from which atomic masses of all nuclides are measured, thus, its atomic mass is exactly 12 daltons by definition. Carbon-12 is composed of 6 protons, 6 neutrons, and 6 electrons. History Before 1959, both the IUPAP and IUPAC used oxygen to define the mole; the chemists defining the mole as the number of atoms of oxygen which had mass 16 g, the physicists using a similar definition but with the oxygen-16 isotope only. The two organizations agreed in 1959/60 to define the mole as follows. ''Mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 12 gram of carbon 12; its symbol is "mol".'' This was adopted by the CIPM (Inte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon-14
Carbon-14, C-14, or radiocarbon, is a radioactive isotope of carbon with an atomic nucleus containing 6 protons and 8 neutrons. Its presence in organic materials is the basis of the radiocarbon dating method pioneered by Willard Libby and colleagues (1949) to date archaeological, geological and hydrogeological samples. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California. Its existence had been suggested by Franz N. D. Kurie, Franz Kurie in 1934. There are three naturally occurring isotopes of carbon on Earth: carbon-12 (), which makes up 99% of all carbon on Earth; carbon-13 (), which makes up 1%; and carbon-14 (), which occurs in trace amounts, making up about 1 or 1.5 atoms per 1012 atoms of carbon in the atmosphere. Carbon-12 and carbon-13 are both stable, while carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Carbon-14 decays into nitrogen-14 () through bet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Testing
Nuclear weapons tests are experiments carried out to determine nuclear weapons' effectiveness, yield, and explosive capability. Testing nuclear weapons offers practical information about how the weapons function, how detonations are affected by different conditions, and how personnel, structures, and equipment are affected when subjected to nuclear explosions. However, nuclear testing has often been used as an indicator of scientific and military strength. Many tests have been overtly political in their intention; most nuclear weapons states publicly declared their nuclear status through a nuclear test. The first nuclear device was detonated as a test by the United States at the Trinity site in New Mexico on July 16, 1945, with a yield approximately equivalent to 20 kilotons of TNT. The first thermonuclear weapon technology test of an engineered device, codenamed "Ivy Mike", was tested at the Enewetak Atoll in the Marshall Islands on November 1, 1952 (local date), also by the U ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Astronomical Epoch
In astronomy, an epoch or reference epoch is a moment in time used as a reference point for some time-varying astronomical quantity. It is useful for the celestial coordinates or orbital elements of a celestial body, as they are subject to perturbations and vary with time. These time-varying astronomical quantities might include, for example, the mean longitude or mean anomaly of a body, the node of its orbit relative to a reference plane, the direction of the apogee or aphelion of its orbit, or the size of the major axis of its orbit. The main use of astronomical quantities specified in this way is to calculate other relevant parameters of motion, in order to predict future positions and velocities. The applied tools of the disciplines of celestial mechanics or its subfield orbital mechanics (for predicting orbital paths and positions for bodies in motion under the gravitational effects of other bodies) can be used to generate an ephemeris, a table of values giving the position ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Google Books
Google Books (previously known as Google Book Search, Google Print, and by its code-name Project Ocean) is a service from Google Inc. that searches the full text of books and magazines that Google has scanned, converted to text using optical character recognition (OCR), and stored in its digital database.The basic Google book link is found at: https://books.google.com/ . The "advanced" interface allowing more specific searches is found at: https://books.google.com/advanced_book_search Books are provided either by publishers and authors through the Google Books Partner Program, or by Google's library partners through the Library Project. Additionally, Google has partnered with a number of magazine publishers to digitize their archives. The Publisher Program was first known as Google Print when it was introduced at the Frankfurt Book Fair in October 2004. The Google Books Library Project, which scans works in the collections of library partners and adds them to the digital invent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrate
In chemistry, a hydrate is a substance that contains water or its constituent elements. The chemical state of the water varies widely between different classes of hydrates, some of which were so labeled before their chemical structure was understood. Chemical nature Inorganic chemistry Hydrates are inorganic salts "containing water molecules combined in a definite ratio as an integral part of the crystal" that are either bound to a metal center or that have crystallized with the metal complex. Such hydrates are also said to contain ''water of crystallization'' or ''water of hydration''. If the water is heavy water in which the constituent hydrogen is the isotope deuterium, then the term ''deuterate'' may be used in place of ''hydrate''. A colorful example is cobalt(II) chloride, which turns from blue to red upon hydration, and can therefore be used as a water indicator. The notation "''hydrated compound''⋅''n''", where ''n'' is the number of water molecules per formula un ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_chloride.jpg)