|
Zinc Phosphate
Zinc phosphate is an inorganic compound with the formula Zn3( PO4)2. This white powder is widely used as a corrosion resistant coating on metal surfaces either as part of an electroplating process or applied as a primer pigment (see also red lead). It has largely displaced toxic materials based on lead or chromium, and by 2006 it had become the most commonly used corrosion inhibitor. Zinc phosphate coats better on a crystalline structure than bare metal, so a seeding agent is often used as a pre-treatment. One common agent is sodium pyrophosphate. Minerals Natural forms of zinc phosphate include minerals hopeite and parahopeite. A somewhat similar mineral is natural hydrous zinc phosphate called tarbuttite, Zn2(PO4)(OH). Both are known from oxidation zones of Zn ore beds and were formed through oxidation of sphalerite by the presence of phosphate-rich solutions. The anhydrous form has not yet been found naturally. Dentistry Zinc phosphate dental cement is one of the oldest and wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Compound
In chemistry, an inorganic compound is typically a chemical compound that lacks carbon–hydrogen bonds, that is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as '' inorganic chemistry''. Inorganic compounds comprise most of the Earth's crust, although the compositions of the deep mantle remain active areas of investigation. Some simple carbon compounds are often considered inorganic. Examples include the allotropes of carbon (graphite, diamond, buckminsterfullerene, etc.), carbon monoxide, carbon dioxide, carbides, and the following salts of inorganic anions: carbonates, cyanides, cyanates, and thiocyanates. Many of these are normal parts of mostly organic systems, including organisms; describing a chemical as inorganic does not necessarily mean that it does not occur within living things. History Friedrich Wöhler's conversion of ammonium cyanate into urea in 1828 is often cited as the starting point of modern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zirconium Dioxide
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral baddeleyite. A dopant stabilized cubic structured zirconia, cubic zirconia, is synthesized in various colours for use as a gemstone and a diamond simulant. Production, chemical properties, occurrence Zirconia is produced by calcining zirconium compounds, exploiting its high thermostability.Ralph Nielsen "Zirconium and Zirconium Compounds" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Structure Three phases are known: monoclinic below 1170 °C, tetragonal between 1170 °C and 2370 °C, and cubic above 2370 °C. The trend is for higher symmetry at higher temperatures, as is usually the case. A small percentage of the oxides of calcium or yttrium stabilize in the cubic phase. The very rare mineral tazheranite, , ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Compounds
Zinc compounds are chemical compounds containing the element zinc which is a member of the group 12 of the periodic table. The oxidation state of zinc in most compounds is the group oxidation state of +2. Zinc may be classified as a post-transition main group element with zinc(II). Zinc compounds are noteworthy for their nondescript behavior, they are generally colorless (unlike other elements with the oxidation number +2, which are usually white), do not readily engage in redox reactions, and generally adopt symmetrical structures. General characteristics In its compounds, Zn2+ ions have an electronic configuration r3d10. As such, Zn2+ tends to have a symmetrical coordination geometry in both its complexes and compounds. In both ZnO and ZnS, (zincblende) zinc is bound tetrahedrally bound to four ligands (oxide and sulfide, respectively). Many complexes, such as ZnCl42−, are tetrahedral. Tetrahedrally coordinated zinc is found in metallo-enzymes such as carbonic anhydras ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphates
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthophosphoric acid . The phosphate or orthophosphate ion is derived from phosphoric acid by the removal of three protons . Removal of one or two protons gives the dihydrogen phosphate ion and the hydrogen phosphate ion ion, respectively. These names are also used for salts of those anions, such as ammonium dihydrogen phosphate and trisodium phosphate. File:3-phosphoric-acid-3D-balls.png, Phosphoricacid File:2-dihydrogenphosphate-3D-balls.png, Dihydrogenphosphate File:1-hydrogenphosphate-3D-balls.png, Hydrogenphosphate File:0-phosphate-3D-balls.png, Phosphate In organic chemistry, phosphate or orthophosphate is an organophosphate, an ester of orthophosphoric acid of the form where one or more hydrogen atoms are replaced by organic groups. An example is trimethyl phosphate, . The term also refers to the tri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glass Ionomer Cement
A glass ionomer cement (GIC) is a dental restorative material used in dentistry as a filling material and luting cement, including for orthodontic bracket attachment. Glass-ionomer cements are based on the reaction of silicate glass-powder (calciumaluminofluorosilicate glass) and polyacrylic acid, an ionomer. Occasionally water is used instead of an acid, altering the properties of the material and its uses. This reaction produces a powdered cement of glass particles surrounded by matrix of fluoride elements and is known chemically as glass polyalkenoate. There are other forms of similar reactions which can take place, for example, when using an aqueous solution of acrylic/ itaconic copolymer with tartaric acid, this results in a glass-ionomer in liquid form. An aqueous solution of maleic acid polymer or maleic/acrylic copolymer with tartaric acid can also be used to form a glass-ionomer in liquid form. Tartaric acid plays a significant part in controlling the setting characterist ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Buffer Solution
A buffer solution (more precisely, pH buffer or hydrogen ion buffer) is an aqueous solution consisting of a mixture of a weak acid and its conjugate base, or vice versa. Its pH changes very little when a small amount of strong acid or base is added to it. Buffer solutions are used as a means of keeping pH at a nearly constant value in a wide variety of chemical applications. In nature, there are many living systems that use buffering for pH regulation. For example, the bicarbonate buffering system is used to regulate the pH of blood, and bicarbonate also acts as a buffer in the ocean. Principles of buffering Buffer solutions resist pH change because of a chemical equilibrium between the weak acid HA and its conjugate base A−: When some strong acid is added to an equilibrium mixture of the weak acid and its conjugate base, hydrogen ions (H+) are added, and the equilibrium is shifted to the left, in accordance with Le Chatelier's principle. Because of this, the hydrogen io ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphoric Acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, which is a colourless, odourless, and non- volatile syrupy liquid. It is a major industrial chemical, being a component of many fertilizers. The compound is an acid. Removal of all three ions gives the phosphate ion . Removal of one or two protons gives dihydrogen phosphate ion , and the hydrogen phosphate ion , respectively. Phosphoric acid forms esters, called organophosphates. The name "orthophosphoric acid" can be used to distinguish this specific acid from other "phosphoric acids", such as pyrophosphoric acid. Nevertheless, the term "phosphoric acid" often means this specific compound; and that is the current IUPAC nomenclature. Production Phosphoric acid is produced industrially by one of two routes, wet processes and dry. We ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from ''magnesia negra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a model sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc Oxide
Zinc oxide is an inorganic compound with the formula . It is a white powder that is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, food supplements, rubbers, plastics, ceramics, glass, cement, lubricants, paints, ointments, adhesives, sealants, pigments, foods, batteries, ferrites, fire retardants, and first-aid tapes. Although it occurs naturally as the mineral zincite, most zinc oxide is produced synthetically. ZnO is a wide-band gap semiconductor of the II-VI semiconductor group. The native doping of the semiconductor due to oxygen vacancies or zinc interstitials is n-type. Other favorable properties include good transparency, high electron mobility, wide band gap, and strong room-temperature luminescence. Those properties make ZnO valuable for a variety of emerging applications: transparent electrodes in liquid crystal displays, energy-saving or heat-protecting windows, and electronics as thin-film transistors and lig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temporary Restoration
Temporary restoration is a temporary filling of tooth preparation, a prepared tooth until permanent restoration is carried out. It is used to cover the prepared part of the tooth, in order to maintain the occlusal space and the contact points, and insulation of the Pulp (tooth), pulpal tissues and maintenance of the Periodontics, periodontal relationship. Sometimes permanent restoration is not done after tooth preparation; this may be to prepare for indirect restoration such as inlays and onlays. Temporary fillings are also used for 'stabilization' techniques where many restorations are needed, and the problem may become worse before it can be fully treated – so temporary fillings are placed in order to stop progression. Materials used *Zinc oxide eugenol *Intermediate Restorative Materials *Zinc Phosphate Cement References The White Smiles Dentistry procedures {{Dentistry-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthodontic
Orthodontics is a dentistry specialty that addresses the diagnosis, prevention, management, and correction of mal-positioned teeth and jaws, and misaligned bite patterns. It may also address the modification of facial growth, known as dentofacial orthopedics. Abnormal alignment of the teeth and jaws is very common. Nearly 50% of the developed world's population, according to the American Association of Orthodontics, has malocclusions severe enough to benefit from orthodontic treatment: although this figure decreases to less than 10% according to the same AAO statement when referring to medically necessary orthodontics. However, conclusive scientific evidence for the health benefits of orthodontic treatment is lacking, although patients with completed orthodontic treatment have reported a higher quality of life than that of untreated patients undergoing orthodontic treatment. Treatment may require several months to a few years, and entails using dental braces and other appliances t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
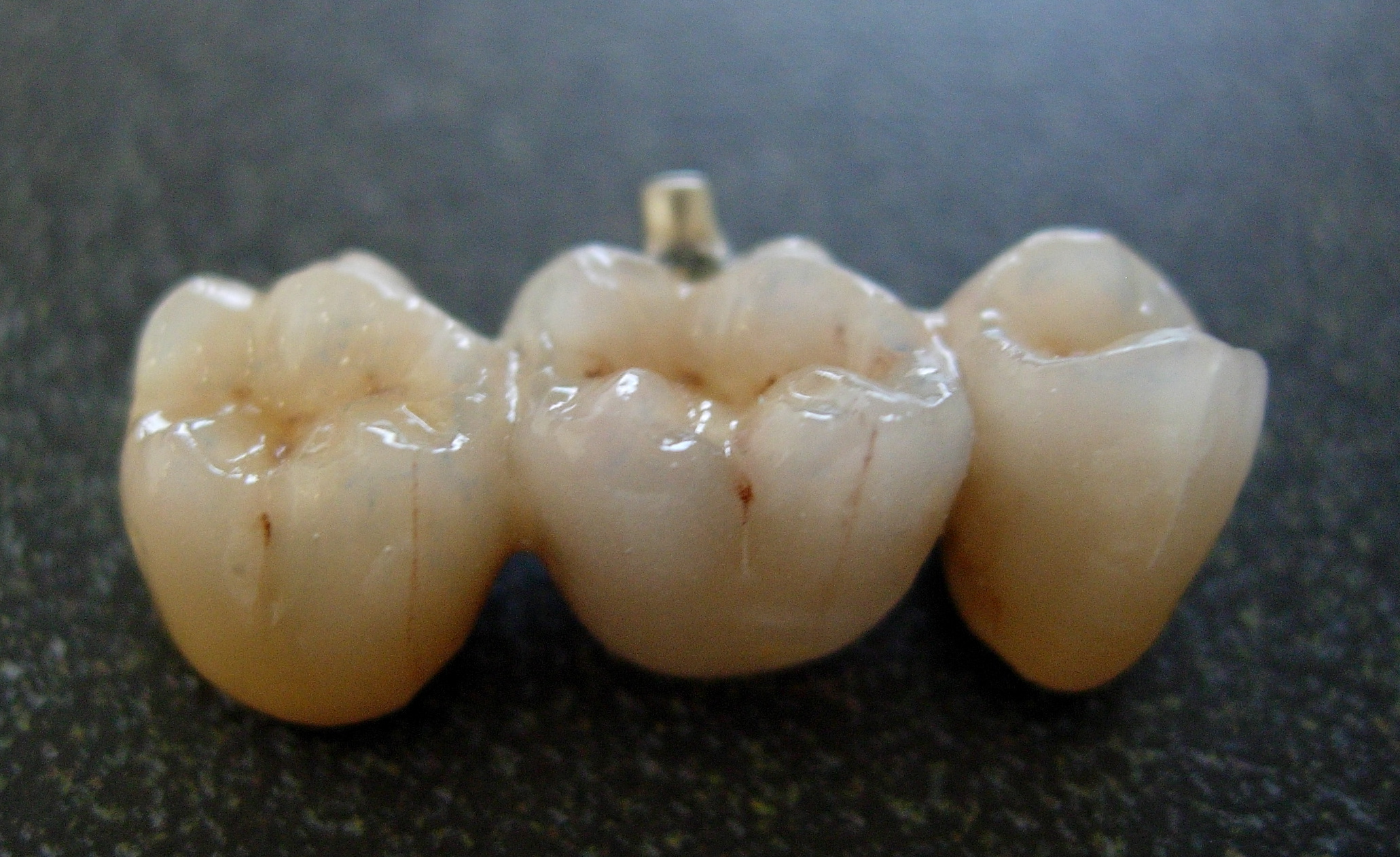
Bridge (dentistry)
A bridge is a fixed dental restoration (a fixed dental prosthesis) used to replace one or more missing teeth by joining an artificial tooth definitively to adjacent teeth or dental implants. Definitions Fixed bridge: A dental prosthesis that is definitively attached to natural teeth and replaces missing teeth. Abutment: The tooth that supports and retains a dental prosthesis. Pontic: The artificial tooth that replaces a missing natural tooth. Retainer: The component attached to the abutment for retention of the prosthesis. Retainers can be major or minor. Unit: Pontics and abutment teeth are referred to as units. The total number of units in a bridge is equal to the number of pontics plus the number of abutment teeth. Saddle: The area on the alveolar ridge which is edentulous where at least one missing tooth is to be reinstated. Connector: Joins the pontic to the retainer or two retainers together. Connectors may be fixed or movable. Span: The length of the alveolar rid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |