|
Zarxio
Filgrastim, sold under the brand name Neupogen among others, is a medication used to treat low neutrophil count. Low neutrophil counts may occur with HIV/AIDS, following chemotherapy or radiation poisoning, or be of an unknown cause. It may also be used to increase white blood cells for gathering during leukapheresis. It is given either by injection into a vein or under the skin. Common side effects include fever, cough, chest pain, joint pain, vomiting, and hair loss. Severe side effects include splenic rupture and allergic reactions. It is unclear if use in pregnancy is safe for the baby. Filgrastim is a recombinant form of the naturally occurring granulocyte colony-stimulating factor (G-CSF). It works by stimulating the body to increase neutrophil production. Filgrastim was approved for medical use in the United States in 1991. It is on the World Health Organization's List of Essential Medicines. Filgrastim biosimilar medications are available. Medical uses Filgrastim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
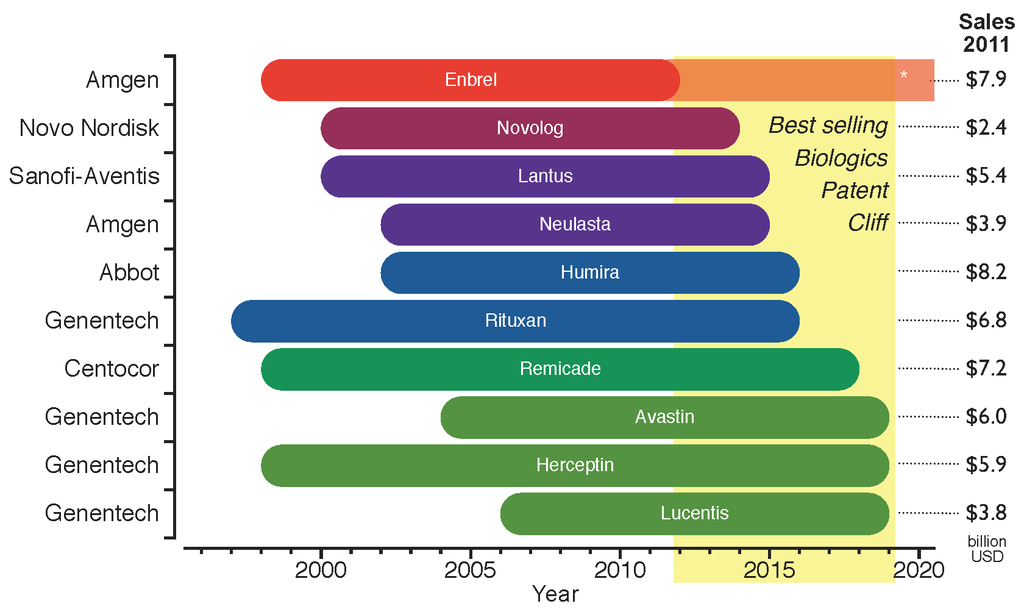
Biosimilar
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. Unlike with generic drugs of the more common small-molecule type, biologics generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite that heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration (FDA), and the Health Products and Food Branch of Health Canada hold the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intraveneous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consump ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hematopoietic Stem Cell Transplantation
Hematopoietic stem-cell transplantation (HSCT) is the transplantation of multipotent hematopoietic stem cells, usually derived from bone marrow, peripheral blood, or umbilical cord blood in order to replicate inside of a patient and to produce additional normal blood cells. It may be autologous (the patient's own stem cells are used), allogeneic (the stem cells come from a donor) or syngeneic (from an identical twin). It is most often performed for patients with certain cancers of the blood or bone marrow, such as multiple myeloma or leukemia. In these cases, the recipient's immune system is usually destroyed with radiation or chemotherapy before the transplantation. Infection and graft-versus-host disease are major complications of allogeneic HSCT. HSCT remains a dangerous procedure with many possible complications; it is reserved for patients with life-threatening diseases. As survival following the procedure has increased, its use has expanded beyond cancer to autoimmun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Febrile Neutropenia
Febrile neutropenia is the development of fever, often with other signs of infection, in a patient with neutropenia, an abnormally low number of neutrophil granulocytes (a type of white blood cell) in the blood. The term neutropenic sepsis is also applied, although it tends to be reserved for patients who are less well. In 50% of cases, an infection is detectable; bacteremia (bacteria in the bloodstream) is present in approximately 20% of all patients with this condition. Signs and symptoms Fever Causes Febrile neutropenia can develop in any form of neutropenia, but is most generally recognized as a complication of chemotherapy when it is myelosuppressive (suppresses the bone marrow from producing blood cells). Diagnosis MASCC and CISNE risk indexes The Multinational Association for Supportive Care in Cancer (MASCC) risk index can be used to identify low-risk patients (score ≥21 points) for serious complications of febrile neutropenia (including death, intensive care unit admis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affordable Care Act
The Affordable Care Act (ACA), formally known as the Patient Protection and Affordable Care Act and colloquially known as Obamacare, is a landmark U.S. federal statute enacted by the 111th United States Congress and signed into law by President Barack Obama on March 23, 2010. Together with the Health Care and Education Reconciliation Act of 2010 amendment, it represents the U.S. healthcare system's most significant regulatory overhaul and expansion of coverage since the enactment of Medicare and Medicaid in 1965. The ACA's major provisions came into force in 2014. By 2016, the uninsured share of the population had roughly halved, with estimates ranging from 20 to 24 million additional people covered. The law also enacted a host of delivery system reforms intended to constrain healthcare costs and improve quality. After it went into effect, increases in overall healthcare spending slowed, including premiums for employer-based insurance plans. The increased coverage was due, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biologics Price Competition And Innovation Act Of 2009
The Biologics Price Competition and Innovation Act of 2009 (BPCI Act) amends the Public Health Service Act (PHS Act) to create an abbreviated approval pathway for biological products shown to be biosimilar to, or interchangeable with, an FDA-licensed reference biological product. The BPCI Act is closely related to the Drug Price Competition and Patent Term Restoration Act of 1984 (or referred to as the "Hatch-Waxman Act The Drug Price Competition and Patent Term Restoration Act (Public Law 98-417), informally known as the Hatch-Waxman Act, is a 1984 United States federal law that encourages the manufacture of generic drugs by the pharmaceutical industry and es ..."), which established abbreviated pathways for the approval of drug products under the Federal Food, Drug, and Cosmetic Act (FD&C Act). References {{Reflist United States federal health legislation United States Public Health Service United States federal patent legislation Biotechnology law Patent law ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
The New York Times
''The New York Times'' (''the Times'', ''NYT'', or the Gray Lady) is a daily newspaper based in New York City with a worldwide readership reported in 2020 to comprise a declining 840,000 paid print subscribers, and a growing 6 million paid digital subscribers. It also is a producer of popular podcasts such as '' The Daily''. Founded in 1851 by Henry Jarvis Raymond and George Jones, it was initially published by Raymond, Jones & Company. The ''Times'' has won 132 Pulitzer Prizes, the most of any newspaper, and has long been regarded as a national " newspaper of record". For print it is ranked 18th in the world by circulation and 3rd in the U.S. The paper is owned by the New York Times Company, which is publicly traded. It has been governed by the Sulzberger family since 1896, through a dual-class share structure after its shares became publicly traded. A. G. Sulzberger, the paper's publisher and the company's chairman, is the fifth generation of the family to head the pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sickle Cell Crisis
Sickle cell disease (SCD) is a group of blood disorders typically inherited from a person's parents. The most common type is known as sickle cell anaemia. It results in an abnormality in the oxygen-carrying protein haemoglobin found in red blood cells. This leads to a rigid, sickle-like shape under certain circumstances. Problems in sickle cell disease typically begin around 5 to 6 months of age. A number of health problems may develop, such as attacks of pain (known as a sickle cell crisis), anemia, swelling in the hands and feet, bacterial infections and stroke. Long-term pain may develop as people get older. The average life expectancy in the developed world is 40 to 60 years. Sickle cell disease occurs when a person inherits two abnormal copies of the β-globin gene (''HBB'') that makes haemoglobin, one from each parent. This gene occurs in chromosome 11. Several subtypes exist, depending on the exact mutation in each haemoglobin gene. An attack can be set off by temper ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemoptysis
Hemoptysis is the coughing up of blood or blood-stained mucus from the bronchi, larynx, trachea, or lungs. In other words, it is the airway bleeding. This can occur with lung cancer, infections such as tuberculosis, bronchitis, or pneumonia, and certain cardiovascular conditions. Hemoptysis is considered massive at . In such cases, there are always severe injuries. The primary danger comes from choking, rather than blood loss. Diagnosis * Past history, history of present illness, family history ** history of tuberculosis, bronchiectasis, chronic bronchitis, mitral stenosis, etc. ** history of cigarette smoking, occupational diseases by exposure to silica dust, etc. * Blood ** duration, frequency, amount ** Amounts of blood: large amounts of blood, or is there blood-streaked sputum ** Probable source of bleeding: Is the blood coughed up, or vomited? * Bloody sputum ** color, characters: blood-streaked, fresh blood, frothy pink, bloody gelatinous. * Accompanying symptoms ** feve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
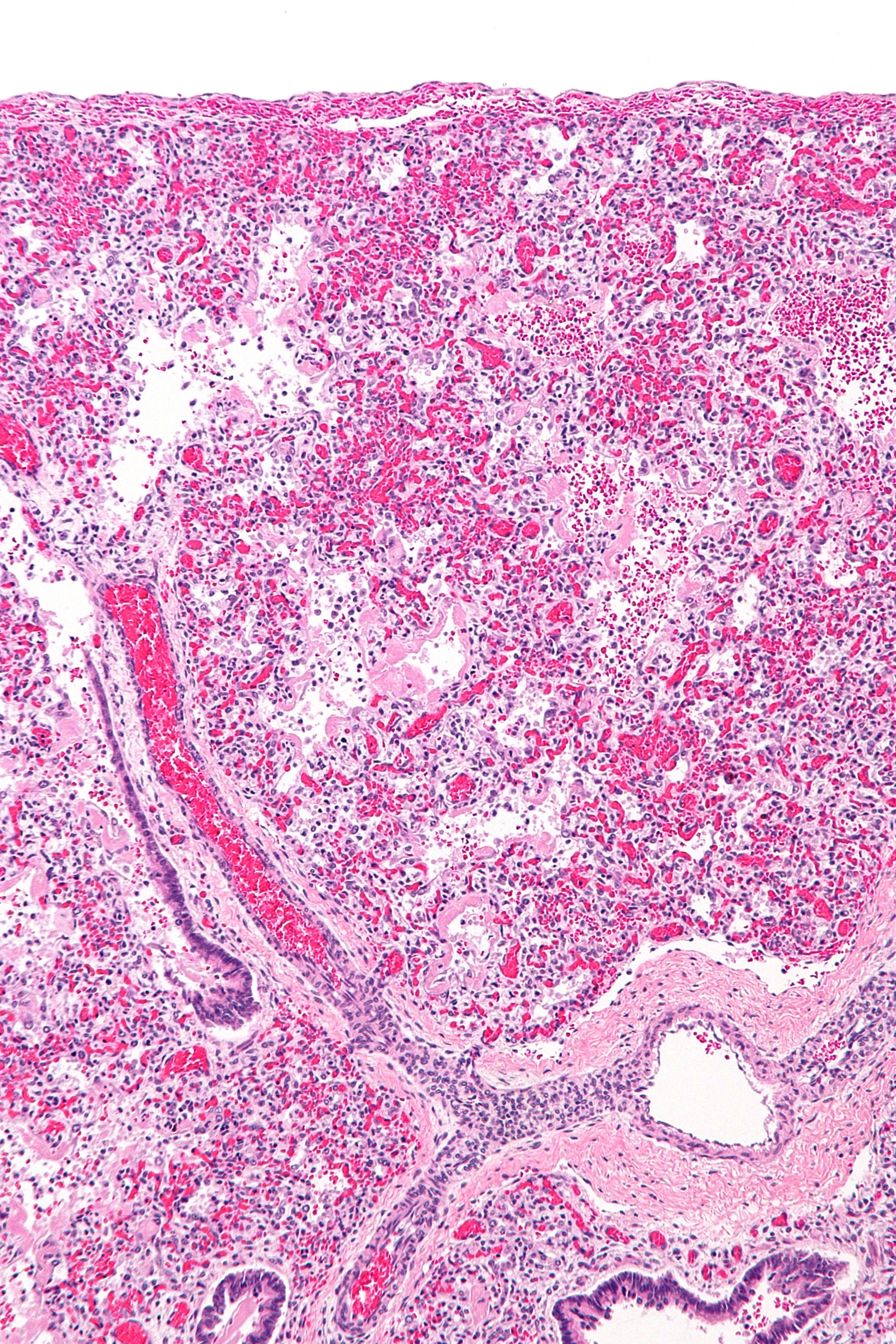
Acute Respiratory Distress Syndrome
Acute respiratory distress syndrome (ARDS) is a type of respiratory failure characterized by rapid onset of widespread inflammation in the lungs. Symptoms include shortness of breath (dyspnea), rapid breathing (tachypnea), and bluish skin coloration (cyanosis). For those who survive, a decreased quality of life is common. Causes may include sepsis, pancreatitis, trauma, pneumonia, and aspiration. The underlying mechanism involves diffuse injury to cells which form the barrier of the microscopic air sacs of the lungs, surfactant dysfunction, activation of the immune system, and dysfunction of the body's regulation of blood clotting. In effect, ARDS impairs the lungs' ability to exchange oxygen and carbon dioxide. Adult diagnosis is based on a PaO2/FiO2 ratio (ratio of partial pressure arterial oxygen and fraction of inspired oxygen) of less than 300 mm Hg despite a positive end-expiratory pressure (PEEP) of more than 5 cm H2O. Cardiogenic pulmonary edema, a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.png)
.jpg)