|
XLR-11 (drug)
XLR-11 (5"-fluoro-UR-144 or 5F-UR-144) is a drug that acts as a potent agonist for the cannabinoid receptors CB1 and CB2 with EC50 values of 98 nM and 83 nM, respectively. It is a 3-(tetramethylcyclopropylmethanoyl)indole derivative related to compounds such as UR-144, A-796,260 and A-834,735, but it is not specifically listed in the patent or scientific literature alongside these other similar compounds, and appears to have not previously been made by Abbott Laboratories, despite falling within the claims of patent WO 2006/069196. XLR-11 was found to produce rapid, short-lived hypothermic effects in rats at doses of 3 mg/kg and 10 mg/kg, suggesting that it is of comparable potency to APICA and STS-135. Detection A forensic standard for this compound is available, and a representative mass spectrum has been posted on Forendex. Recreational use XLR-11 was instead first identified by laboratories in 2012 as an ingredient in synthetic cannabis smoking blends, and appe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology From the Greek αγωνιστής (agōnistēs), contestant; champion; rival < αγων (agōn), contest, combat; exertion, struggle < αγω (agō), I lead, lead towards, conduct; drive Types of agonists can be activated by either endogenous agonists (such as |
Acute Kidney Injury
Acute kidney injury (AKI), previously called acute renal failure (ARF), is a sudden decrease in kidney function that develops within 7 days, as shown by an increase in serum creatinine or a decrease in urine output, or both. Causes of AKI are classified as either prerenal (due to decreased blood flow to the kidney), intrinsic renal (due to damage to the kidney itself), or postrenal (due to blockage of urine flow). Prerenal causes of AKI include sepsis, dehydration, excessive blood loss, cardiogenic shock, heart failure, cirrhosis, and certain medications like ACE inhibitors or NSAIDs. Intrinsic renal causes of AKI include glomerulonephritis, lupus nephritis, acute tubular necrosis, certain antibiotics, and chemotherapeutic agents. Postrenal causes of AKI include kidney stones, bladder cancer, neurogenic bladder, enlargement of the prostate, narrowing of the urethra, and certain medications like anticholinergics. The diagnosis of AKI is made based on a person's signs and sympto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indoles
Indole is an aromatic heterocyclic organic compound with the formula C8 H7 N. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indole is widely distributed in the natural environment and can be produced by a variety of bacteria. As an intercellular signal molecule, indole regulates various aspects of bacterial physiology, including spore formation, plasmid stability, resistance to drugs, biofilm formation, and virulence. The amino acid tryptophan is an indole derivative and the precursor of the neurotransmitter serotonin. General properties and occurrence Indole is a solid at room temperature. It occurs naturally in human feces and has an intense fecal odor. At very low concentrations, however, it has a flowery smell, and is a constituent of many perfumes. It also occurs in coal tar. The corresponding substituent is called indolyl. Indole undergoes electrophilic substitution, mainly at position 3 (see diagram i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organofluorides
Organofluorine chemistry describes the chemistry of the organofluorines, organic compounds that contain the carbon–fluorine bond. Organofluorine compounds find diverse applications ranging from Lipophobicity, oil and hydrophobe, water repellents to pharmaceuticals, refrigerants, and reagents in catalysis. In addition to these applications, some organofluorine compounds are pollutants because of their contributions to ozone depletion, global warming, bioaccumulation, and toxicity. The area of organofluorine chemistry often requires special techniques associated with the handling of fluorinating agents. The carbon–fluorine bond Fluorine has several distinctive differences from all other substituents encountered in organic molecules. As a result, the physical and chemical properties of organofluorines can be distinctive in comparison to other organohalogens. # The carbon–fluorine bond is one of the strongest in organic chemistry (an average bond energy around 480 kJ/molKirsch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Designer Drugs
A designer drug is a structural or functional analog of a controlled substance that has been designed to mimic the pharmacological effects of the original drug, while avoiding classification as illegal and/or detection in standard drug tests. Designer drugs include psychoactive substances that have been designated by the European Union as new psychoactive substances (NPS) as well as analogs of performance-enhancing drugs such as designer steroids. Some of these were originally synthesized by academic or industrial researchers in an effort to discover more potent derivatives with fewer side effects, and shorter duration (and possibly also because it is easier to apply for patents for new molecules) and were later co-opted for recreational use. Other designer drugs were prepared for the first time in clandestine laboratories. Because the efficacy and safety of these substances have not been thoroughly evaluated in animal and human trials, the use of some of these drugs may result i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoids
Cannabinoids () are several structural classes of compounds found in the cannabis plant primarily and most animal organisms (although insects lack such receptors) or as synthetic compounds. The most notable cannabinoid is the phytocannabinoid tetrahydrocannabinol (THC) (delta-9-THC), the primary intoxicating compound in cannabis. Cannabidiol (CBD) is a major constituent of temperate Cannabis plants and a minor constituent in tropical varieties. At least 113 distinct phytocannabinoids have been isolated from cannabis, although only four (i.e., THCA, CBDA, CBCA and their common precursor CBGA) have been demonstrated to have a biogenetic origin. It was reported in 2020 that phytocannabinoids can be found in other plants such as rhododendron, licorice and liverwort, and earlier in Echinacea. Phytocannabinoids are multi-ring phenolic compounds structurally related to THC, but endocannabinoids are fatty acid derivatives. Nonclassical synthetic cannabinoids (cannabimimetics) include amin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
XLR-12
XLR-12 is an indole-based synthetic cannabinoid drug that was invented by Abbott Laboratories in 2006. It is an analogue of XLR-11 where the 5-fluoropentyl chain has been replaced with a 4,4,4-trifluorobutyl chain. XLR-12 is relatively highly selective for the CB2 receptor, with a Ki of 0.09 nM and 167x selectivity over the related CB1 receptor, however it still retains appreciable affinity for CB1 with a Ki of 15 nM. Legal status XLR-12 is illegal in Hungary and Japan. See also * UR-144 * FUB-144 * JTE 7-31 JTE 7-31 is a selective cannabinoid receptor agonist invented by Japan Tobacco. It is a reasonably highly selective CB2 agonist, but still retains appreciable affinity at CB1, with a Ki of 0.088nM at CB2 vs 11nM at CB1. Legality JTE 7-31 is il ... References Cannabinoids Designer drugs Tetramethylcyclopropanoylindoles {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JWH-018
JWH-018 (1-pentyl-3-(1-naphthoyl)indole, NA-PIMO or AM-678) is an analgesic chemical from the naphthoylindole family that acts as a full agonist at both the CB1 and CB2 cannabinoid receptors, with some selectivity for CB2. It produces effects in animals similar to those of tetrahydrocannabinol (THC), a cannabinoid naturally present in cannabis, leading to its use in synthetic cannabis products that in some countries are sold legally as "incense blends". As a full agonist at both the CB1 and CB2 cannabinoid receptors, this chemical compound is classified as an analgesic medication. The analgesic effects of cannabinoid ligands, mediated by CB1 receptors are well established in treatment of neuropathic pain, as well as cancer pain and arthritis. These compounds work by mimicking the body's naturally-produced endocannabinoid hormones such as 2-AG and anandamide (AEA), which are biologically active and can exacerbate or inhibit nerve signaling. As the cause is poorly understood in chr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FAB-144
FAB-144 is an indazole-based synthetic cannabinoid that is presumed to be a potent agonist of the CB1 receptor and has been sold online as a designer drug. It is the indazole analogue of XLR-11. Legal status Sweden's public health agency suggested classifying FAB-144 as a hazardous substance on November 10, 2014. See also * JWH-018 * STS-135 * UR-144 UR-144 (TMCP-018, KM-X1, MN-001, YX-17) is a drug invented by Abbott Laboratories, that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor. Pharmacology ... References Cannabinoids Designer drugs Organofluorides Indazoles Cyclopropanes {{cannabinoid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
AM-2201
AM-2201 (1-(5-fluoropentyl)-3-(1-naphthoyl)indole) is a recreational designer drug that acts as a potent but nonselective full agonist for the cannabinoid receptor. It is part of the AM series of cannabinoids discovered by Alexandros Makriyannis at Northeastern University. Hazards Convulsions have been reported including at doses as low as 10 mg. Pharmacology AM-2201 is a full agonist for cannabinoid receptors. Affinities are: with a ''K''i of 1.0 nM at CB1 and 2.6 nM at CB2. The 4-methyl functional analog MAM-2201 probably has similar affinities. AM-2201 has an EC50 of 38 nM for human CB1 receptors, and 58 nM for human CB2 receptors. AM-2201 produces bradycardia and hypothermia in rats at doses of 0.3–3 mg/kg, comparable to the potency of JWH-018 in rats, suggesting potent cannabinoid-like activity. Pharmacokinetics AM-2201 metabolism differs only slightly from that of JWH-018. AM-2201 ''N''-dealkylation produces fluoropentane instead of p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Temporary Class Drug
A temporary class drug is a relatively new status for controlled drugs, which has been adopted in some jurisdictions, notably New Zealand and the United Kingdom, to attempt to bring newly synthesised designer drugs under legal control. The controlled drug legislation in these jurisdictions requires drug scheduling decisions to follow an evidence-based process, where the harms of the drug are assessed and reviewed so that an appropriate legal status can be assigned. Since many designer drugs sold in recent years have had little or no published research that could help inform such a decision, they have been widely sold as "legal highs", often for months, before sufficient evidence accumulates to justify placing them on the controlled drug schedules. This situation has been deemed to be undesirable, as every time a designer drug has been banned, novel compounds with similar effects have been quickly developed and brought to market, often with worse health consequences reported than the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
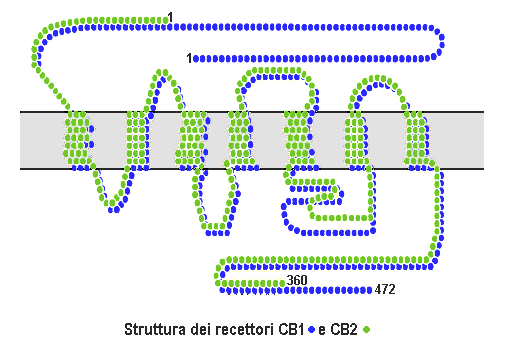
Cannabinoid Receptor
Cannabinoid receptors, located throughout the body, are part of the endocannabinoid system a class of cell membrane receptors in the G protein-coupled receptor superfamily. As is typical of G protein-coupled receptors, the cannabinoid receptors contain seven transmembrane spanning domains. Cannabinoid receptors are activated by three major groups of ligands: endocannabinoids; plant cannabinoids (such as Tetrahydrocannabinol, produced by the cannabis plant); and synthetic cannabinoids (such as HU-210). All of the endocannabinoids and phytocannabinoids (plant based cannabinoids) are lipophilic. There are two known subtypes of cannabinoid receptors, termed CB1 and CB2. The CB1 receptor is expressed mainly in the brain (central nervous system or "CNS"), but also in the lungs, liver and kidneys. The CB2 receptor is expressed mainly in the immune system, in hematopoietic cells, and in parts of the brain. The protein sequences of CB1 and CB2 receptors are about 44% similar. When ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |