|
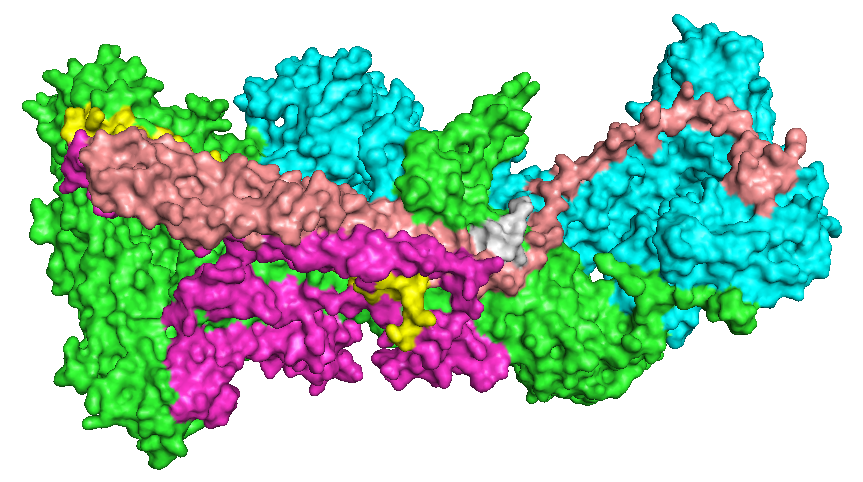
WAVE Regulatory Complex
The WAVE regulatory complex (WRC, SCAR complex) is a five-subunit protein complex in the Wiskott-Aldrich syndrome protein (WASP) family involved in the formation of the actin cytoskeleton through interaction with the Arp2/3 complex. The holocomplex comprises WAVE1 (also known as WASF1), CYFIP1, ABI2, Nap1 and HSPC300 in its canonical form, or orthologues of these. Composition The proteins within the WRC form a CYFIP1-Nap1 heterodimer and a WAVE1-Abi2-HSPC300 heterotrimer, and following interaction with Rac1, the holocomplex has been observed in a CYFIP1-Nap1-Abi2 heterotrimer subcomplex and an active WAVE1-HSPC300 heterodimer subcomplex. Function WRC recruitment to the sites of actin nucleation events at the cell periphery is mediated by the binding of a number of ligands containing a conserved WRC interacting receptor sequence (WIRS) which binds to a conserved location shared across the surfaces of Abi2 and CYFIP1. The WRC is activated by interaction with the Rac1 (via ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimer (chemistry)
In chemistry, a trimer (; ) is a molecule or polyatomic anion formed by combination or association of three molecules or ions of the same substance. In technical jargon, a trimer is a kind of oligomer derived from three identical precursors often in competition with polymerization. Examples Alkyne trimerisation In 1866, Marcellin Berthelot reported the first example of cyclotrimerization, the conversion of acetylene to benzene. This process was commercialized: : Nitrile trimerization Symmetrical 1,3,5-triazines are prepared by trimerization of certain nitriles such as cyanogen chloride or cyanimide. Cyanogen chloride and cyanogen bromide each trimerize at elevated temperatures over a carbon catalyst. The chloride gives cyanuric chloride: : The bromide has an extended shelflife when refrigerated. Like the chloride, it undergoes ab exothermic trimerisation to form cyanuric bromide. This reaction is catalyzed by traces of bromine, metal salts, acids and bases. For this reason ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARL1
ADP-ribosylation factor-like protein 1 is a protein that in humans is encoded by the ''ARL1'' gene. Function The protein encoded by this gene belongs to the ARL (ADP-ribosylation factor-like) family of proteins, which are structurally related to ADP-ribosylation factors (ARFs). ARFs, described as activators of cholera toxin (CT) ADP-ribosyltransferase activity, regulate intracellular vesicular membrane trafficking, and stimulate a phospholipase D (PLD) isoform. Although, ARL proteins were initially thought not to activate CT or PLD, later work showed that they are weak stimulators of PLD and CT in a phospholipid dependent manner. Interactions ARL1 has been shown to interact with GOLGA4 and GOLGA1 Golgin subfamily A member 1 is a protein that in humans is encoded by the ''GOLGA1'' gene. The Golgi apparatus, which participates in glycosylation and transport of proteins and lipids in the secretory pathway, consists of a series of stacked cis .... References External lin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARF6
ADP-ribosylation factor 6 (ARF6) is a member of the ADP ribosylation factor family of GTP-binding proteins. ARF6 has a variety of cellular functions that are frequently involved in trafficking of biological membranes and transmembrane protein cargo. ARF6 has specifically been implicated in endocytosis of plasma membrane proteins and also, to a lesser extent, plasma membrane protein recycling. Function This gene encodes a member of the human ARF gene family, which is part of the Ras superfamily. The ARF genes encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking and as activators of phospholipase D. The product of this gene is localized to the plasma membrane, and regulates vesicular trafficking, remodelling of membrane lipids, and signaling pathways that lead to actin remodeling. A pseudogene of this gene is located on chromosome 7. ARF6 can interact with βarrestin upo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARF5
ADP-ribosylation factor 5 is a protein that in humans is encoded by the ''ARF5'' gene. ADP-ribosylation factor 5 (ARF5) is a member of the human ARF gene family. These genes encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking and as activators of phospholipase D. The gene products include 6 ARF proteins and 11 ARF-like proteins and constitute 1 family of the RAS superfamily. The ARF proteins are categorized as class I (ARF1, ARF2, and ARF3), class II (ARF4 and ARF5) and class III (ARF6). The members of each class share a common gene organization. The ARF5 gene spans approximately 3.2kb of genomic DNA and contains six exons and five introns. Interactions ARF5 has been shown to interact with ARFIP2 Arfaptin-2 is a protein that in humans is encoded by the ''ARFIP2'' gene. Interactions ARFIP2 has been shown to interact with Arf6 ADP-ribosylation factor 6 (ARF6) is a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ARF1
ADP-ribosylation factor 1 is a protein that in humans is encoded by the ''ARF1'' gene. Function ADP-ribosylation factor 1 (ARF1) is a member of the human ARF gene family. The family members encode small guanine nucleotide-binding proteins that stimulate the ADP-ribosyltransferase activity of cholera toxin and play a role in vesicular trafficking as activators of phospholipase D. The gene products, including 6 ARF proteins and 11 ARF-like proteins, constitute a family of the RAS superfamily. The ARF proteins are categorized as class I (ARF1, ARF2 and ARF3), class II (ARF4 and ARF5) and class III (ARF6), and members of each class share a common gene organization. The ARF1 protein is localized to the Golgi apparatus and has a central role in intra-Golgi transport. Multiple alternatively spliced transcript variants encoding the same protein have been found for this gene. The major mechanism of action of Brefeldin A is through inhibition of ARF1. Interactions ARF1 has been shown ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small GTPase
Small GTPases (), also known as small G-proteins, are a family of hydrolase enzymes that can bind and hydrolyze guanosine triphosphate (GTP). They are a type of G-protein found in the cytosol that are homologous to the alpha subunit of heterotrimeric G-proteins, but unlike the alpha subunit of G proteins, a small GTPase can function independently as a hydrolase enzyme to bind to and hydrolyze a guanosine triphosphate (GTP) to form guanosine diphosphate (GDP). The best-known members are the Ras GTPases and hence they are sometimes called Ras subfamily GTPases. A typical G-protein is active when bound to GTP and inactive when bound to GDP (i.e. when the GTP is hydrolyzed to GDP). The GDP can be then replaced by free GTP. Therefore, a G-protein can be switched on and off. GTP hydrolysis is accelerated by GTPase activating proteins (GAPs), while GTP exchange is catalyzed by guanine nucleotide exchange factors (GEFs). Activation of a GEF typically activates its cognate G-protein, whi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
ADP Ribosylation Factor
ADP ribosylation factors (ARFs) are members of the ARF family of GTP-binding proteins of the Ras superfamily. ARF family proteins are ubiquitous in eukaryotic cells, and six highly conserved members of the family have been identified in mammalian cells. Although ARFs are soluble, they generally associate with membranes because of N-terminus myristoylation. They function as regulators of vesicular traffic and actin remodelling. The small ADP ribosylation factor (Arf) GTP-binding proteins are major regulators of vesicle biogenesis in intracellular traffic. They are the founding members of a growing family that includes Arl (Arf-like), Arp (Arf-related proteins) and the remotely related Sar (Secretion-associated and Ras-related) proteins. Arf proteins cycle between inactive GDP-bound and active GTP-bound forms that bind selectively to effectors. The classical structural GDP/GTP switch is characterised by conformational changes at the so-called switch 1 and switch 2 regions, whic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lifetime. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Actin
Actin is a family of globular multi-functional proteins that form microfilaments in the cytoskeleton, and the thin filaments in muscle fibrils. It is found in essentially all eukaryotic cells, where it may be present at a concentration of over 100 μM; its mass is roughly 42 kDa, with a diameter of 4 to 7 nm. An actin protein is the monomeric subunit of two types of filaments in cells: microfilaments, one of the three major components of the cytoskeleton, and thin filaments, part of the contractile apparatus in muscle cells. It can be present as either a free monomer called G-actin (globular) or as part of a linear polymer microfilament called F-actin (filamentous), both of which are essential for such important cellular functions as the mobility and contraction of cells during cell division. Actin participates in many important cellular processes, including muscle contraction, cell motility, cell division and cytokinesis, vesicle and organelle movement, cell sign ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |