|
Ugi's Amine
Ugi’s amine is a chemical compound named for the chemist who first reported its synthesis in 1970, Ivar Karl Ugi, Ivar Ugi. It is a ferrocene derivative (chemistry), derivative. Since its first report, Ugi’s amine has found extensive use as the synthetic precursor to a large number of metal Ligand, ligands that bear planar chirality. These ligands have since found extensive use in a variety of catalytic reactions. The compound may exist in either the 1''S'' or 1''R'' isomer, both of which have synthetic utility and are commercially available. Most notably, it is the synthetic precursor to the Josiphos ligands, Josiphos class of ligands. History In 1967, Schlӧg repurposed the term “planar chirality” for use in substituted ferrocene terminology, which is necessary for ferrocenes in which ferrocene's innate plane of symmetry is broken by introducing two substituents to one of its ring. Nozaki, ''et al.'' demonstrated that a ferrocene derivative bearing a chiral amine subst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Compound
A chemical compound is a chemical substance composed of many identical molecules (or molecular entities) containing atoms from more than one chemical element held together by chemical bonds. A molecule consisting of atoms of only one element is therefore not a compound. A compound can be transformed into a different substance by a chemical reaction, which may involve interactions with other substances. In this process, bonds between atoms may be broken and/or new bonds formed. There are four major types of compounds, distinguished by how the constituent atoms are bonded together. Molecular compounds are held together by covalent bonds; ionic compounds are held together by ionic bonds; intermetallic compounds are held together by metallic bonds; coordination complexes are held together by coordinate covalent bonds. Non-stoichiometric compounds form a disputed marginal case. A chemical formula specifies the number of atoms of each element in a compound molecule, using the s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinyl Acetate
Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate and ethene-vinyl acetate copolymers, important industrial polymers. Production The worldwide production capacity of vinyl acetate was estimated at 6,969,000 tonnes/year in 2007, with most capacity concentrated in the United States (1,585,000 all in Texas), China (1,261,000), Japan (725,000) and Taiwan (650,000). The average list price for 2008 was US$1600/tonne. Celanese is the largest producer (ca 25% of the worldwide capacity), while other significant producers include China Petrochemical Corporation (7%), Chang Chun Group (6%), and LyondellBasell (5%). It is a key ingredient in furniture glue. Preparation Vinyl acetate is the acetate ester of vinyl alcohol. Since vinyl alcohol is highly unstable (with respect to acetaldehyde), the preparation of vinyl acetate is more complex than the synthesis of other acetate esters. The major industrial route i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tridentate Ligand
A tridentate ligand (or terdentate ligand) is a ligand that has three atoms that can function as acceptor atoms in a coordination complex. Well-known tridentate ligands include diethylenetriamine with three nitrogen donor atoms, and the iminodiacetate anion which consists of one deprotonated amine nitrogen and a pair of carboxylate groups. An octahedrally coordinated atom has six positions around it. Two tridentate ligands may form a complex with such an atom. There are two possible arrangements for such a complex: ''fac'' where the coordination is in a triangle on one face of the octahedron, and ''mer'' where the coordinating atoms are in an arc around the central atom, with two atoms of the ligand opposite each other. ''Fac'' tridentate ligands are termed scorpionate ligands, especially in reference to polypyrazolylborates. If the tridentate ligand is not symmetrical, then in the ''fac'' complexes in octahedral coordination there are three possible isomers. In the ''mer'' com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
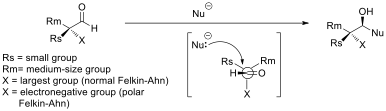
Asymmetric Induction
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis. Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist. Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homogeneous Catalysis
In chemistry, homogeneous catalysis is catalysis by a soluble catalyst in a solution. Homogeneous catalysis refers to reactions where the catalyst is in the same phase as the reactants, principally in solution. In contrast, heterogeneous catalysis describes processes where the catalysts and substrate are in distinct phases, typically solid-gas, respectively. The term is used almost exclusively to describe solutions and implies catalysis by organometallic compounds. Homogeneous catalysis is an established technology that continues to evolve. An illustrative major application is the production of acetic acid. Enzymes are examples of homogeneous catalysts. Examples Acid catalysis The proton is a pervasive homogeneous catalyst because water is the most common solvent. Water forms protons by the process of self-ionization of water. In an illustrative case, acids accelerate (catalyze) the hydrolysis of esters: :CH3CO2CH3 + H2O CH3CO2H + CH3OH At neutral pH, aqueous solutions of most e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substitution Of Ugi's Amine
Substitution may refer to: Arts and media * Chord substitution, in music, swapping one chord for a related one within a chord progression *Substitution (poetry), a variation in poetic scansion * "Substitution" (song), a 2009 song by Silversun Pickups *Substitution (theatre), an acting methodology *Tritone substitution, in music, reinterpreting a chord via a new root note located an augmented fourth or diminished fifth distant from the root of the original interpretation Science and mathematics Biology and chemistry *Base-pair substitution or point mutation, a type of mutation * Substitution reaction, where a functional group in a chemical compound is replaced by another group *Substitution, a process in which an allele arises and undergoes fixation Mathematics and computing *Substitution (algebra), replacing occurrences of some symbol by a given value * Substitution (logic), a syntactic transformation on strings of symbols of a formal language *String substitution, a mapping of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Iodide
Iodomethane, also called methyl iodide, and commonly abbreviated "MeI", is the chemical compound with the formula CH3I. It is a dense, colorless, volatile liquid. In terms of chemical structure, it is related to methane by replacement of one hydrogen atom by an atom of iodine. It is naturally emitted by rice plantations in small amounts. It is also produced in vast quantities estimated to be greater than 214,000 tons annually by algae and kelp in the world's temperate oceans, and in lesser amounts on land by terrestrial fungi and bacteria. It is used in organic synthesis as a source of methyl groups. Preparation and handling Iodomethane is formed via the exothermic reaction that occurs when iodine is added to a mixture of methanol with red phosphorus. The iodinating reagent is phosphorus triiodide that is formed ''in situ:'' :3 CH3OH + PI3 → 3 CH3I + H2PO3H Alternatively, it is prepared from the reaction of dimethyl sulfate with potassium iodide in the presence of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithiation Of Ugi's Amine
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom to the substrates in synthetic steps, through nucleophilic addition or simple deprotonation. Organolithium reagents are used in industry as an initiator for anionic polymerization, which leads to the production of various elastomers. They have also been applied in asymmetric synthesis in the pharmaceutical industry. Due to the large difference in electronegativity between the carbon atom and the lithium atom, the C−Li bond is highly ionic. Owing to the polar nature of the C−Li bond, organolithium reagents are good nucleophiles and strong bases. For laboratory organic synthesis, many organolithium reagents are commercially available in solution form. These reagents are highly reactive, and are sometimes pyrophoric. History and de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetra-n-butylammonium Fluoride
Tetra-''n''-butylammonium fluoride, commonly abbreviated to TBAF and ''n''-Bu4NF, is a quaternary ammonium salt with the chemical formula (CH3CH2CH2CH2)4N+F−. It is commercially available as the white solid trihydrate and as a solution in tetrahydrofuran. TBAF is used as a source of fluoride ion in organic solvents. Preparation and properties TBAF can be prepared by passing hydrofluoric acid through an ion-exchange resin, followed by tetrabutylammonium bromide. Upon evaporation of the water, TBAF can be collected as an oil in quantitative yield. Preparing anhydrous samples is of interest as the basicity of fluoride increases by more than 20 p''K'' units on passing from aqueous to aprotic solvent. However, heating samples of the hydrated material to 77 °C under vacuum causes decomposition to the hydrogen difluoride salt. Similarly, samples dried at 40 °C under high vacuum still contain 10-30 mol% of water and some 10% of difluoride. Instead, anhydrous TBAF ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclopentadienyl
{{Chemistry index ...
Cyclopentadienyl can refer to *Cyclopentadienyl anion, or cyclopentadienide, **Cyclopentadienyl ligand *Cyclopentadienyl radical, • *Cyclopentadienyl cation, See also *Pentadienyl In organic chemistry, pentadienyl refers to the organic radical, anion, or cation with the formula , where ''z'' = 0, −1, +1, respectively. Organometallic chemistry In organometallic chemistry, the pentadienyl anion is a ligand, the acyclic ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steric Interaction
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivity of ions and molecules. Steric effects complement electronic effects, which dictate the shape and reactivity of molecules. Steric repulsive forces between overlapping electron clouds result in structured groupings of molecules stabilized by the way that opposites attract and like charges repel. Steric hindrance Steric hindrance is a consequence of steric effects. Steric hindrance is the slowing of chemical reactions due to steric bulk. It is usually manifested in ''intermolecular reactions'', whereas discussion of steric effects often focus on ''intramolecular interactions''. Steric hindrance is often exploited to control selectivity, such as slowing unwanted side-reactions. Steric hindrance between adjacent groups can also affect torsiona ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TMSCl
Trimethylsilyl chloride, also known as chlorotrimethylsilane is an organosilicon compound (silyl halide), with the formula (CH3)3SiCl, often abbreviated Me3SiCl or TMSCl. It is a colourless volatile liquid that is stable in the absence of water. It is widely used in organic chemistry. Preparation TMSCl is prepared on a large scale by the ''direct process'', the reaction of methyl chloride with a silicon-copper alloy. The principal target of this process is dimethyldichlorosilane, but substantial amounts of the trimethyl and monomethyl products are also obtained. The relevant reactions are (Me = CH3): : x MeCl + Si → Me3SiCl, Me2SiCl2, MeSiCl3, other products Typically about 2–4% of the product stream is the monochloride, which forms an azeotrope with MeSiCl3. Reactions and uses TMSCl is reactive toward nucleophiles, resulting in the replacement of the chloride. In a characteristic reaction of TMSCl, the nucleophile is water, resulting in hydrolysis to give the hexamethy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |