|
Uplizna
Inebilizumab, sold under the brand name Uplizna, is a medication for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults. The most common adverse reactions include urinary tract infection, headache, joint pain (arthralgia), nausea and back pain. Inebilizumab is a humanized mAb that binds to and depletes CD19+ B cells including plasmablasts and plasma cells. The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication. Medical uses Inebilizumab is indicated for the treatment of neuromyelitis optica spectrum disorder (NMOSD) in adults with a particular antibody (patients who are anti-aquaporin-4 or AQP4 antibody positive). NMOSD is a rare autoimmune disorder in which immune system cells and autoantibodies attack and damage the optic nerves and spinal cord. NMOSD can be associated with antibodies that bind to a protein called aquaporin-4 (AQP4). Binding of the anti-AQP4 antibody appears to activate other components of the i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thomas Tedder
Thomas Fletcher Tedder, Ph.D., (born May 14, 1956) is an American immunologist. He is best known for his work in the fields of B lymphocyte biology and regulation. He is currently the Alter E. Geller Professor for Research in Immunology at Duke University. Career Tedder received his Ph.D. in molecular cell biology from the University of Alabama in 1984 and completed his postdoctoral training as a research fellow in pathology at Harvard Medical School. He was a faculty member at Dana–Farber Cancer Institute and Harvard Medical School Harvard Medical School (HMS) is the graduate medical school of Harvard University and is located in the Longwood Medical Area of Boston, Massachusetts. Founded in 1782, HMS is one of the oldest medical schools in the United States and is consi ... from 1985 to 1998 before joining Duke University in 1993 as its founding chairman of immunology. Tedder studies the structure and function of B lymphocyte cell surface molecules that regulate B cell fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
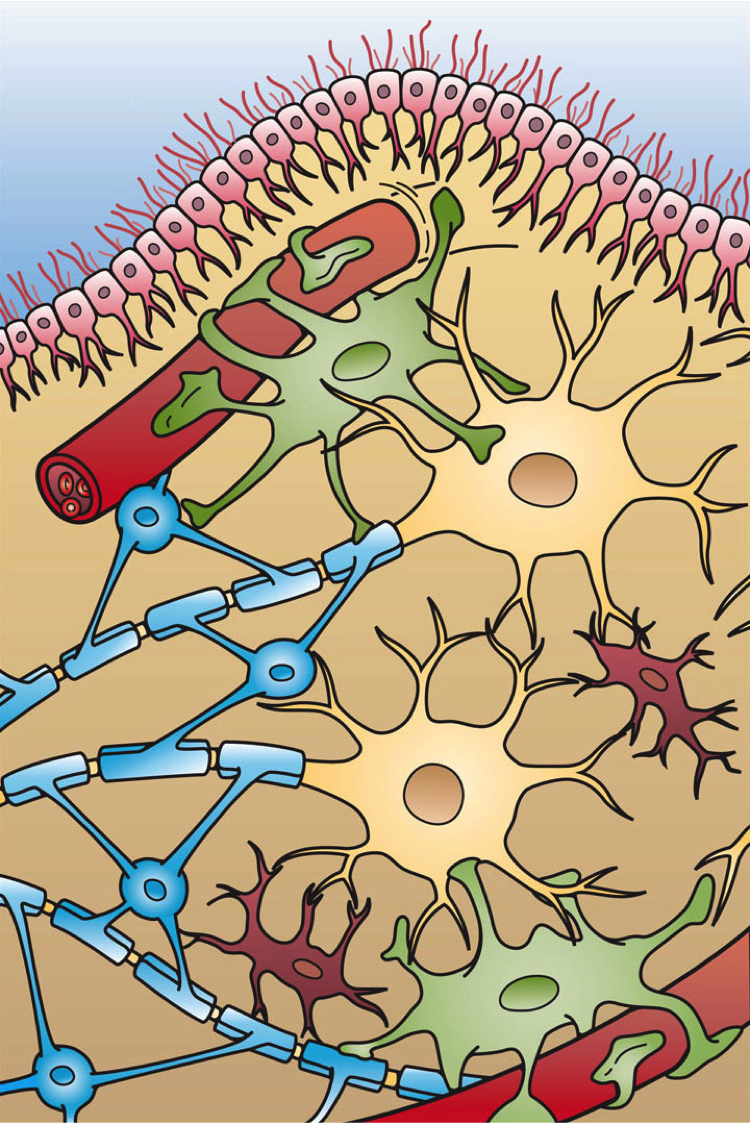
Neuromyelitis Optica
Neuromyelitis optica spectrum disorders (NMOSD), including neuromyelitis optica (NMO), are autoimmune diseases characterized by acute inflammation of the optic nerve (optic neuritis, ON) and the spinal cord (myelitis). Episodes of ON and myelitis can be simultaneous or successive. A relapsing disease course is common, especially in untreated patients. In more than 80% of cases, NMO is caused by immunoglobulin G Autoantibody, autoantibodies to aquaporin 4 (anti-AQP4 diseases, anti-AQP4), the most abundant Aquaporin, water channel protein in the central nervous system. A subset of anti-AQP4-negative cases is associated with antibodies against myelin oligodendrocyte glycoprotein (Anti-MOG associated encephalomyelitis, anti-MOG). Rarely, NMO may occur in the context of other autoimmune diseases (e.g. Connective tissue disease, connective tissue disorders, paraneoplastic syndromes) or infectious diseases. In some cases, the etiology remains unknown (Idiopathic disease, idiopathic NMO). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD19
B-lymphocyte antigen CD19, also known as CD19 molecule ( Cluster of Differentiation 19), B-Lymphocyte Surface Antigen B4, T-Cell Surface Antigen Leu-12 and CVID3 is a transmembrane protein that in humans is encoded by the gene ''CD19''. In humans, CD19 is expressed in all B lineage cells. Contrary to some early doubts, human plasma cells do express CD19, as confirmed by others. CD19 plays two major roles in human B cells: on the one hand, it acts as an adaptor protein to recruit cytoplasmic signaling proteins to the membrane; on the other, it works within the CD19/CD21 complex to decrease the threshold for B cell receptor signaling pathways. Due to its presence on all B cells, it is a biomarker for B lymphocyte development, lymphoma diagnosis and can be utilized as a target for leukemia immunotherapies. Structure In humans, CD19 is encoded by the 7.41 kilobase ''CD19'' gene located on the short arm of chromosome 16. It contains at least fifteen exons, four that encode extrac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intravenous
Intravenous therapy (abbreviated as IV therapy) is a medical technique that administers fluids, medications and nutrients directly into a person's vein. The intravenous route of administration is commonly used for rehydration or to provide nutrients for those who cannot, or will not—due to reduced mental states or otherwise—consume food or water by mouth. It may also be used to administer medications or other medical therapy such as blood products or electrolytes to correct electrolyte imbalances. Attempts at providing intravenous therapy have been recorded as early as the 1400s, but the practice did not become widespread until the 1900s after the development of techniques for safe, effective use. The intravenous route is the fastest way to deliver medications and fluid replacement throughout the body as they are introduced directly into the circulatory system and thus quickly distributed. For this reason, the intravenous route of administration is also used for the consumpti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antineoplastic Agent
Chemotherapy (often abbreviated to chemo and sometimes CTX or CTx) is a type of cancer treatment that uses one or more anti-cancer drugs (list of chemotherapeutic agents, chemotherapeutic agents or alkylating agents) as part of a standardized chemotherapy regimen. Chemotherapy may be given with a cure, curative intent (which almost always involves combinations of drugs) or it may aim to prolong life or to Palliative care, reduce symptoms (Palliative care, palliative chemotherapy). Chemotherapy is one of the major categories of the medical discipline specifically devoted to pharmacotherapy for cancer, which is called oncology#Specialties, ''medical oncology''. The term ''chemotherapy'' has come to connote non-specific usage of intracellular poisons to inhibit mitosis (cell division) or induce DNA damage (naturally occurring), DNA damage, which is why inhibition of DNA repair can augment chemotherapy. The connotation of the word chemotherapy excludes more selective agents that bl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C), but the agency also enforces other laws, notably Section 361 of the Public Health Service Act, as well as associated regulations. Much of this regulatory-enforcement work is not d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
First-in-class Medication
A first-in-class medication is a pharmaceutical that uses a "new and unique mechanism of action" to treat a particular medical condition. While the Food and Drug Administration's Center for Drug Evaluation and Research tracks first-in-class medications and reports on them annually, first-in-class is not considered a regulatory category. Although many first-in-class medications qualify as breakthrough therapies, Regenerative Medicine Advanced Therapies and/or orphan drugs, first-in-class status itself has no regulatory effect. Examples Controversy Safety By definition, a first-in-class drug does not have the safety evidence from analogous products that not-first-in-class drugs would have. However, a study investigating recalls and warnings in relation to first-in-class drugs approved between 1997 and 2012 by Health Canada has found that first-in-class drugs actually have a more favourable benefit-to-harm ratio. Economics First-in-class drugs are often seen as commerci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
MedImmune
MedImmune, LLC was a wholly owned subsidiary of AstraZeneca before February 14, 2019, when it was announced that the MedImmune name and branding would be discontinued in favor of AstraZeneca. MedImmune was founded in 1988 as Molecular Vaccines, Inc, and was purchased in 2007 for $15.6 billion. Its main offices were located in Gaithersburg, MD, Cambridge, UK, and Mountain View, CA. It produced '' Synagis'', a drug for the prevention of respiratory infections in infants, which accounted for US$ 1.06 billion of its US$ 1.2 billion in revenue for 2005, and ''FluMist'', a nasal spray influenza vaccine introduced in 2004. MedImmune acquired ''FluMist'' when it purchased Aviron in 2002 for US$ 1.5 billion. ''FluMist'' sales totaled US$ 104 million in 2008, US$ 54.8 million in 2007, and US$ 36.4 million in 2006. ''FluMist'' was approved for children two years of age and older in 2007, but initially was approved only for healthy people ages 5 to 49, a significant limitation because it e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orphan Drug
An orphan drug is a pharmaceutical agent developed to treat medical conditions which, because they are so rare, would not be profitable to produce without government assistance. The conditions are referred to as orphan diseases. The assignment of orphan status to a disease and to drugs developed to treat it is a matter of public policy in many countries and has yielded medical breakthroughs that might not otherwise have been achieved, due to the economics of drug research and development. In the U.S. and the EU, it is easier to gain marketing approval for an orphan drug. There may be other financial incentives, such as an extended period of exclusivity, during which the producer has sole rights to market the drug. All are intended to encourage development of drugs which would otherwise lack sufficient profit motive to attract corporate research budgets and personnel. Definition According to the US Food and Drug Administration (FDA), an orphan drug is defined as one "intended for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Committee For Medicinal Products For Human Use
The Committee for Medicinal Products for Human Use (CHMP), formerly known as Committee for Proprietary Medicinal Products (CPMP), is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding medicinal products for human use. See also * Committee for Medicinal Products for Veterinary Use The Committee for Medicinal Products for Veterinary Use (CVMP) is the European Medicines Agency's committee responsible for elaborating the agency's opinions on all issues regarding veterinary medicines. Text was copied from this source which is © ... References External links Committee for Medicinal Products for Human Use (CHMP) Health and the European Union {{eu-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Medicines Agency
The European Medicines Agency (EMA) is an agency of the European Union (EU) in charge of the evaluation and supervision of medicinal products. Prior to 2004, it was known as the European Agency for the Evaluation of Medicinal Products or European Medicines Evaluation Agency (EMEA).Set up by EC Regulation No. 2309/93 as the European Agency for the Evaluation of Medicinal Products, and renamed by EC Regulation No. 726/2004 to the European Medicines Agency, it had the acronym EMEA until December 2009. The European Medicines Agency does not call itself EMA either – it has no official acronym but may reconsider if EMA becomes commonly accepted (secommunication on new visual identity an). The EMA was set up in 1995, with funding from the European Union and the pharmaceutical industry, as well as indirect subsidy from member states, its stated intention to harmonise (but not replace) the work of existing national medicine regulatory bodies. The hope was that this plan would not onl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


.jpg)
