|
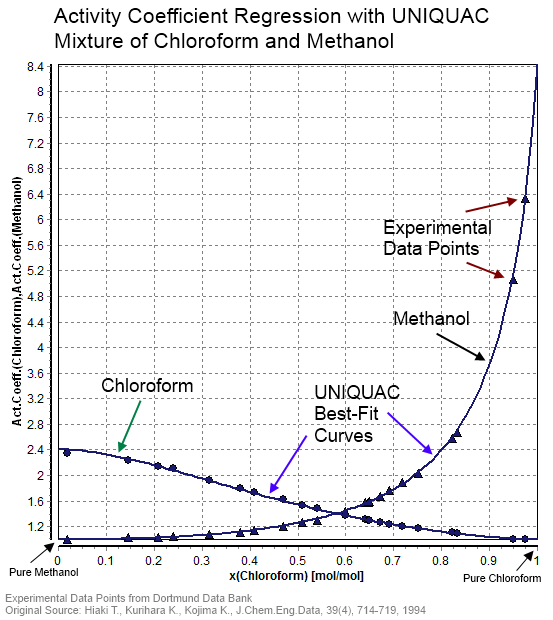
UNIQUAC
In statistical thermodynamics, UNIQUAC (a portmanteau of universal quasichemical) is an activity coefficient model used in description of phase equilibria. The model is a so-called lattice model and has been derived from a first order approximation of interacting molecule surfaces. The model is, however, not fully thermodynamically consistent due to its two- liquid mixture approach. In this approach the local concentration around one central molecule is assumed to be independent from the local composition around another type of molecule. The UNIQUAC model can be considered a second generation activity coefficient because its expression for the excess Gibbs energy consists of an entropy term in addition to an enthalpy term. Earlier activity coefficient models such as the Wilson equation and the non-random two-liquid model (NRTL model) only consist of enthalpy terms. Today the UNIQUAC model is frequently applied in the description of phase equilibria (i.e. liquid–solid, liquid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COSMOSPACE
COSMOSPACE (COSMO Surface-Pair Activity Coefficient Equation) is an activity coefficient model in which the activity coefficient of the components in a liquid chemical mixture can be related through their molar fraction. It was initially developed as an implicit solution to COSMO-RS. While UNIQUAC is a first order approximation, COSMOSPACE gives the exact solution of a lattice model in which pairwise molecule surfaces interact. Therefore, COSMOSPACE outperforms Uniquac in the description of vapor–liquid and liquid–liquid phase equilibria.Thesis of Dennis Bosse, "Diffusion, Viscosity, and Thermodynamics in Liquid Systems" , Technischen Universität Kaiserslautern (2005)" See also * UNIQUAC * :fr:UNIQUAC, UNIQUAC * COSMOSPACE *Chemical equilibrium *Chemical thermodynamics *Fugacity In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activity Coefficient
In thermodynamics, an activity coefficient is a factor used to account for deviation of a mixture of chemical substances from ideal behaviour. In an ideal mixture, the microscopic interactions between each pair of chemical species are the same (or macroscopically equivalent, the enthalpy change of solution and volume variation in mixing is zero) and, as a result, properties of the mixtures can be expressed directly in terms of simple concentrations or partial pressures of the substances present e.g. Raoult's law. Deviations from ideality are accommodated by modifying the concentration by an ''activity coefficient''. Analogously, expressions involving gases can be adjusted for non-ideality by scaling partial pressures by a fugacity coefficient. The concept of activity coefficient is closely linked to that of activity in chemistry. Thermodynamic definition The chemical potential, \mu_\mathrm, of a substance B in an ideal mixture of liquids or an ideal solution is given by :\mu_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", ''AIChE Journal'', vol. 21 (1975), p. 1086 is a semi-empirical system for the prediction of non-electrolyte activity in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations. The UNIFAC model was first published in 1975 by Fredenslund, Jones and John Prausnitz, a group of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
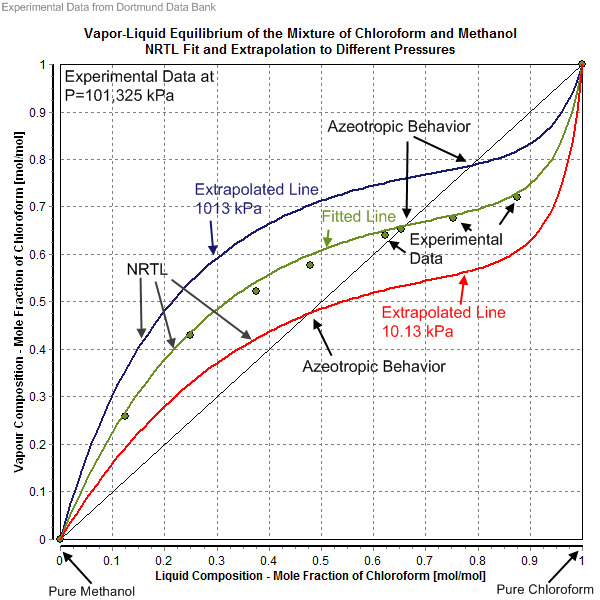
Non-random Two-liquid Model
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson that the local concentration around a molecule is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind U_ and that with the molecules of the other kind U_. The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-composition models are not thermodynamically consistent for a one-fluid model for a real mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Non-random Two-liquid Model
The non-random two-liquid model (abbreviated NRTL model) is an activity coefficient model that correlates the activity coefficients \gamma_i of a compound with its mole fractions x_i in the liquid phase concerned. It is frequently applied in the field of chemical engineering to calculate phase equilibria. The concept of NRTL is based on the hypothesis of Wilson that the local concentration around a molecule is different from the bulk concentration. This difference is due to a difference between the interaction energy of the central molecule with the molecules of its own kind U_ and that with the molecules of the other kind U_. The energy difference also introduces a non-randomness at the local molecular level. The NRTL model belongs to the so-called local-composition models. Other models of this type are the Wilson model, the UNIQUAC model, and the group contribution model UNIFAC. These local-composition models are not thermodynamically consistent for a one-fluid model for a real mi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Free Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work that may be performed by a thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its surroundings, minus the work of the pressure forces. The Gibbs energy is the thermodynamic potential that is minim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vapor–liquid Equilibrium
In thermodynamics and chemical engineering, the vapor–liquid equilibrium (VLE) describes the distribution of a chemical species between the vapor phase and a liquid phase. The concentration of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part of the total gas pressure) if any other gas(es) are present with the vapor. The equilibrium vapor pressure of a liquid is in general strongly dependent on temperature. At vapor–liquid equilibrium, a liquid with individual components in certain concentrations will have an equilibrium vapor in which the concentrations or partial pressures of the vapor components have certain values depending on all of the liquid component concentrations and the temperature. The converse is also true: if a vapor with components at certain concentrations or partial pressures is in vapor–liquid equilibrium with its liquid, then the component concentration ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibbs Energy
In thermodynamics, the Gibbs free energy (or Gibbs energy; symbol G) is a thermodynamic potential that can be used to calculate the maximum amount of work (physics), work that may be performed by a closed system, thermodynamically closed system at constant temperature and pressure. It also provides a necessary condition for processes such as Chemical reaction, chemical reactions that may occur under these conditions. The Gibbs free energy change , measured in joules in International System of Units, SI) is the ''maximum'' amount of non-expansion work that can be extracted from a closed system (one that can exchange heat and work with its surroundings, but not matter) at fixed temperature and pressure. This maximum can be attained only in a completely reversible process (thermodynamics), reversible process. When a system transforms reversibly from an initial state to a final state under these conditions, the decrease in Gibbs free energy equals the work done by the system to its s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution. The extent of the solubility of a substance in a specific solvent is generally measured as the concentration of the solute in a saturated solution, one in which no more solute can be dissolved. At this point, the two substances are said to be at the solubility equilibrium. For some solutes and solvents, there may be no such limit, in which case the two substances are said to be " miscible in all proportions" (or just "miscible"). The solute can be a solid, a liquid, or a gas, while the solvent is usually solid or liquid. Both may be pure substances, or may themselves be solutions. Gases are always miscible in all proportions, except in very extreme situations,J. de Swaan Arons and G. A. M. Diepen (1966): "Gas—Gas Equilibria". ''Journal of Chemical Physics'', ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fugacity
In chemical thermodynamics, the fugacity of a real gas is an effective partial pressure which replaces the mechanical partial pressure in an accurate computation of the chemical equilibrium constant. It is equal to the pressure of an ideal gas which has the same temperature and molar Gibbs free energy as the real gas. Fugacities are determined experimentally or estimated from various models such as a Van der Waals gas that are closer to reality than an ideal gas. The real gas pressure and fugacity are related through the dimensionless fugacity coefficient . \varphi = \frac For an ideal gas, fugacity and pressure are equal and so . Taken at the same temperature and pressure, the difference between the molar Gibbs free energies of a real gas and the corresponding ideal gas is equal to . The fugacity is closely related to the thermodynamic activity. For a gas, the activity is simply the fugacity divided by a reference pressure to give a dimensionless quantity. This reference press ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |