|
Tripodal Ligand
Tripodal ligands are tri- and tetradentate ligands. They are popular in research in the areas of coordination chemistry and homogeneous catalysis. Because the ligands are polydentate, they do not readily dissociate from the metal centre. Many tripodal ligands have C3 symmetry. Coordination chemistry In their coordination complexes with an octahedral molecular geometry the tridentate tripod ligands occupy one face, leading to a fixed facial (or ''fac'') geometry. The tetradentate tripodal ligands occupy four contiguous sites, leaving two ''cis'' positions available on the octahedral metal center. When bound to four- and five-coordinate metal centres, these ligands impose C3 symmetry, which can lead to uncommon ligand field splitting patterns. Tripodal ligands are often able to coordinately saturate metal ions with lower coordination numbers. One tripodal ligand of commercial significance is nitrilotriacetate, N(CH2CO2−)3 because it is cheaply produced and has a high affinity f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-aminoethyl)amine
Tris(2-aminoethyl)amine is the organic compound with the chemical formula, formula N(CH2CH2NH2)3. This colourless liquid is soluble in water and is highly basic, consisting of a tertiary amine center and three pendant primary amine groups. Abbreviated tren or TREN it is a crosslinking agent in the synthesis of polyimine networks and a tripodal ligand in coordination chemistry. Tren is a C3-symmetric, tetradentate chelating ligand that forms stable complexes with transition metals, especially those in the 2+ and 3+ oxidation states. Tren complexes exist with relatively few isomers, reflecting the constrained connectivity of this tetramine. Thus, only a single achiral stereoisomer exists for [Co(tren)X2]+, where X is halide or pseudohalide. In contrast, for [Co(trien)X2]+ five diastereomers are possible, four of which are chiral. In a few cases, tren serves as a tridentate ligand with one of the primary amine groups non-coordinated. Tren is a common impurity in the more common trie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cis,cis-1,3,5-Triaminocyclohexane
''cis'',''cis''-1,3,5-Triaminocyclohexane is an organic compound with the formula (CH2CHNH2)3. It is a triamine. Of the many isomers possible for triaminocyclohexane, the ''cis'',''cis''-1,3,5-derivative has attracted attention because it is a common tripodal ligand, abbreviated as tach. It is a colorless oil. It is a popular tridentate ligand in coordination chemistry. It is prepared from the triscarbamate of cyclohexane. The latter is generated via the Curtius rearrangement The Curtius rearrangement (or Curtius reaction or Curtius degradation), first defined by Theodor Curtius in 1885, is the thermal decomposition of an acyl azide to an isocyanate with loss of nitrogen gas. The isocyanate then undergoes attack by a va ... starting from cyclohexanetricarboxylic acid. Related ligands * Tris(aminomethyl)ethane, another tripodal triamine (CH3C(CH2NH2]3) References {{DEFAULTSORT:Triaminocyclohexane, cis,cis-1,3,5- Tripodal ligands Polyamines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-Tris(aminomethyl)ethane
1,1,1-Tris(aminomethyl)ethane (TAME) is an organic compound with the formula CHC(CHNH). It is a colorless liquid. It is classified as a polyamine tripodal ligand, i.e., capable of binding to metal ions through three sites and hence is a tridentate chelating ligand, occupying a face of the coordination polyhedron. Preparation TAME is synthesized by the Pd/C-catalyzed hydrogenation of 1,1,1-tris(azidomethyl)ethane. Although azides are potentially explosive, they are excellent and practical source of primary amines. The required tris(azidomethyl)ethane is obtained from the tritosylate by salt metathesis using sodium azide. These two steps are: :3 NaN + CHC(CHOTs) → CHC(CHN) + 3 NaOTs :3 H + CHC(CHN) → CHC(CHNH) + 3 N Complexes of TAME The tripodal TAME ligand coordinates facially to metal ions. This stereochemical feature has been exploited in the preparation of platinum(IV) cage complexes, e.g., t(tame) which is a six coordinate Pt(IV) complex. Platinum in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kläui Ligand
The Kläui ligand is the anion −. The ligand, popularized by Wolfgang Kläui, binds metals and metalloids via a facial O3 donor set. Related tridentate and tripodal anionic ligands include trispyrazolylborates. : 160px, General structure of a metal center coordinated to a κ3-Kläui ligand. The ligand is derived from the cationic complex of trimethylphosphite 2+ via an Arbuzov reaction. Using other phosphites and other cyclopentadienyl ligands, a large variety of derivatives are possible. The parent acid H is highly soluble in water (270 g/100 mL). Its p''K''a is about 2. Many complexes have been described, including bis(chelate Chelation is a type of bonding of ions and molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These ligands are ...) complexes of the type {M 3}2.html" ;"title="CH3O)2PO.html" ;"title="(C5H5)Co[(CH3O)2PO">(C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trispyrazolylborate
In inorganic chemistry, the trispyrazolylborate ligand, abbreviated Tp−, is an anionic tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion B(C3N2H3)3sup>−, but the term trispyrazolylborate refers to derivatives substituted at on the pyrazolyl rings. This family of compounds are sometimes called scorpionate ligands. Tp ligands As suggested by the resonance structures, the nitrogen centers that are not bonded to boron are basic. These centers bind to three adjacent sites of a metal such that the simple adducts have C3v symmetry. The facial bonding mode is reminiscent of cyclopentadienyl ligands, although the ligand field stabilization energy of Tp− is weaker as indicated by the fact that Fe(Tp)2 is a spin-crossover complex whereas ferrocene is low-spin. The Tp ligands are usually prepared from the reaction of pyrazole with potassium borohydride: :KBH4 + 3 C3H3N2H → K B(C3N2H3)3 + 3H2 Intermediates include the monopyrazolylborate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioinorganic Chemistry
Bioinorganic chemistry is a field that examines the role of metals in biology. Bioinorganic chemistry includes the study of both natural phenomena such as the behavior of metalloproteins as well as artificially introduced metals, including those that are non-essential, in medicine and toxicology. Many biological processes such as respiration depend upon molecules that fall within the realm of inorganic chemistry. The discipline also includes the study of inorganic models or mimics that imitate the behaviour of metalloproteins. As a mix of biochemistry and inorganic chemistry, bioinorganic chemistry is important in elucidating the implications of electron-transfer proteins, substrate bindings and activation, atom and group transfer chemistry as well as metal properties in biological chemistry. The successful development of truly interdisciplinary work is necessary to advance bioinorganic chemistry. Composition of living organisms About 99% of mammals' mass are the elements carb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(2-pyridylmethyl)amine
Tris(2-pyridylmethyl)amine (abbreviated TPMA or TPA) is an organic compound with the formula (C5H4NCH2)3N. It is a tertiary amine with three picolyl substituents. It is a white solid that is soluble in polar organic solvents. It is a ligand in coordination chemistry. The ligand is prepared by the alkylation of 2-picolylamine by picolyl chloride:{{cite book , author1=James W. Canary , author2=Yihan Wang , author3=Richard Roy, Jr. , title = Tris 2-Pyridyl)MethylAmine (TPA) and (+)-Bis 2-Pyridyl)methyl1-(2-Pyridyl)-Ethylamine (α-Metpa) , journal = Inorg. Synth. , series=Inorganic Syntheses , year = 1998 , volume = 32 , pages = 70–75 , doi = 10.1002/9780470132630.ch11, isbn=9780470132630 :2 C5H4NCH2Cl + C5H4NCH2NH2 → (C5H4NCH2)3N + 2 HCl TPMA is a tripodal ligand, often used to simulate the coordination environment within some proteins. It is also used as a copper ligand in ATRP. Related ligands * dipicolylamine, an intermediate in the synthesis of TPMA. *2-pi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
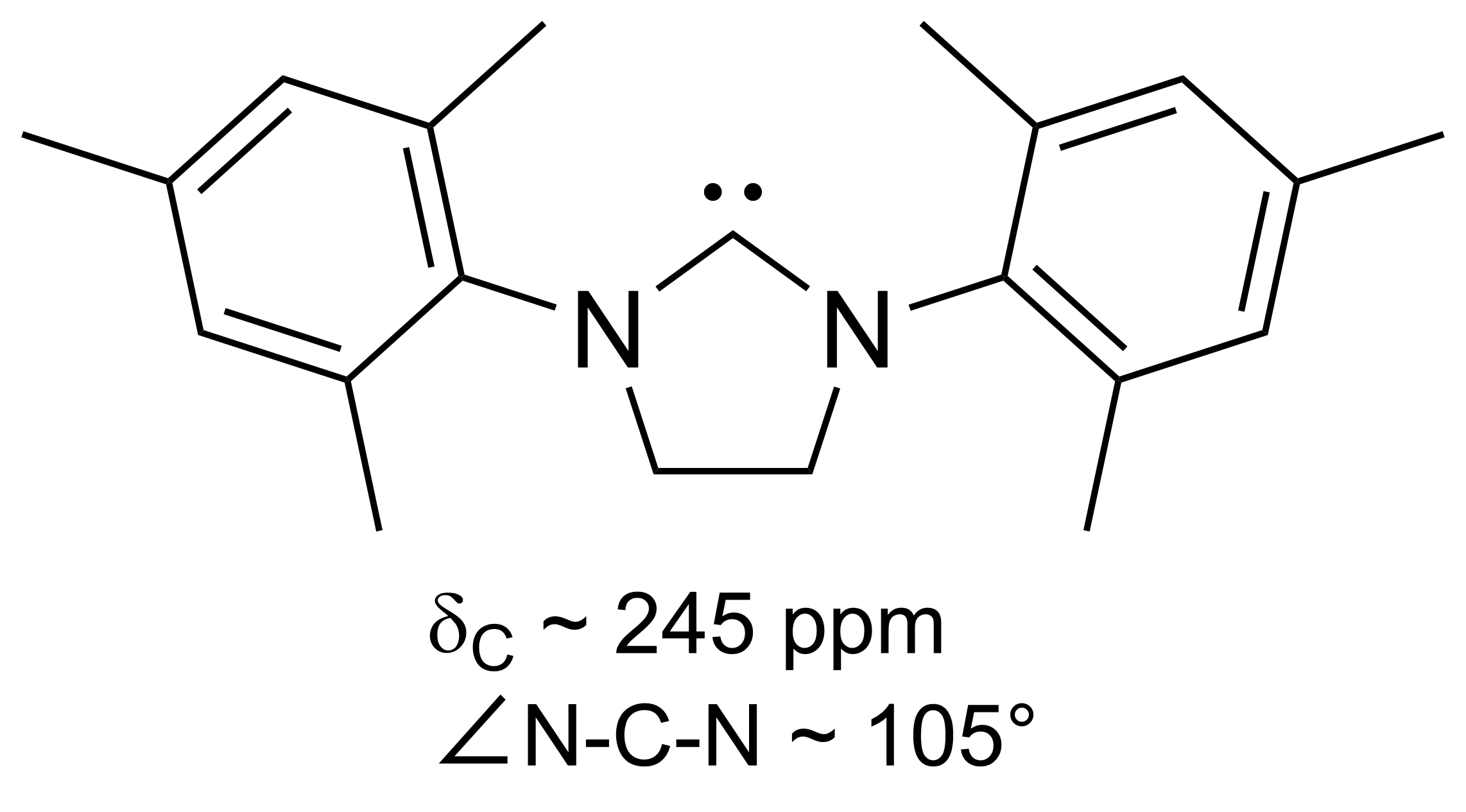
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
1,1,1-Tris(diphenylphosphinomethyl)ethane
1,1,1-Tris(diphenylphosphinomethyl)ethane, also called Triphos, is an organophosphorus compound with the formula CH3C H2PPh2sub>3. An air-sensitive white solid, it is a tripodal ligand ("three-legged") of idealized C3v symmetry. It was originally prepared by the reaction of sodium diphenylphosphide and CH3C(CH2Cl)3: :3 Ph2PNa + CH3C(CH2Cl)3 → CH3C H2PPh2sub>3 + 3 NaCl It forms complexes with many transition metals, usually as a tripodal ligand. Such complexes are used to analyze mechanistic aspects of homogeneous catalysts. For example, rhodium forms complexes with CH3C H2PPh2sub>3 like triphos)RhCl(C2H4) triphos)RhH(C2H4) and triphos)Rh(C2H5)(C2H4) provide model intermediates in the catalytic cycle for hydrogenation of alkenes. Triphos sometimes behaves as a bidentate ligand. Illustrative cases include ''fac''- n(CO)3Br(η2-triphos)and (CO)4(η2-triphos) where M is Cr, Mo, or W. Triphos serves as a tridentate-bridging ligand in an icosahedral In geometry, an i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrilotriacetic Acid
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid that is used as a chelating agent, which forms coordination compounds with metal ions (chelates) such as Ca2+, Co2+, Cu2+, and Fe3+. Production and use Nitrilotriacetic acid is commercially available as the free acid and as the sodium salt. It is produced from ammonia, formaldehyde, and sodium cyanide or hydrogen cyanide. Worldwide capacity is estimated at 100 thousand tonnes per year. NTA is also cogenerated as an impurity in the synthesis of EDTA, arising from reactions of the ammonia coproduct.Hart, J. Roger (2005) "Ethylenediaminetetraacetic Acid and Related Chelating Agents" in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim. Older routes to NTA included alkylation of ammonia with chloroacetic acid and oxidation of triethanolamine. Coordination chemistry and applications NTA is a tripodal tetradentate trianionic ligand. The uses of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |