|
Triphenyltin Hydroxide
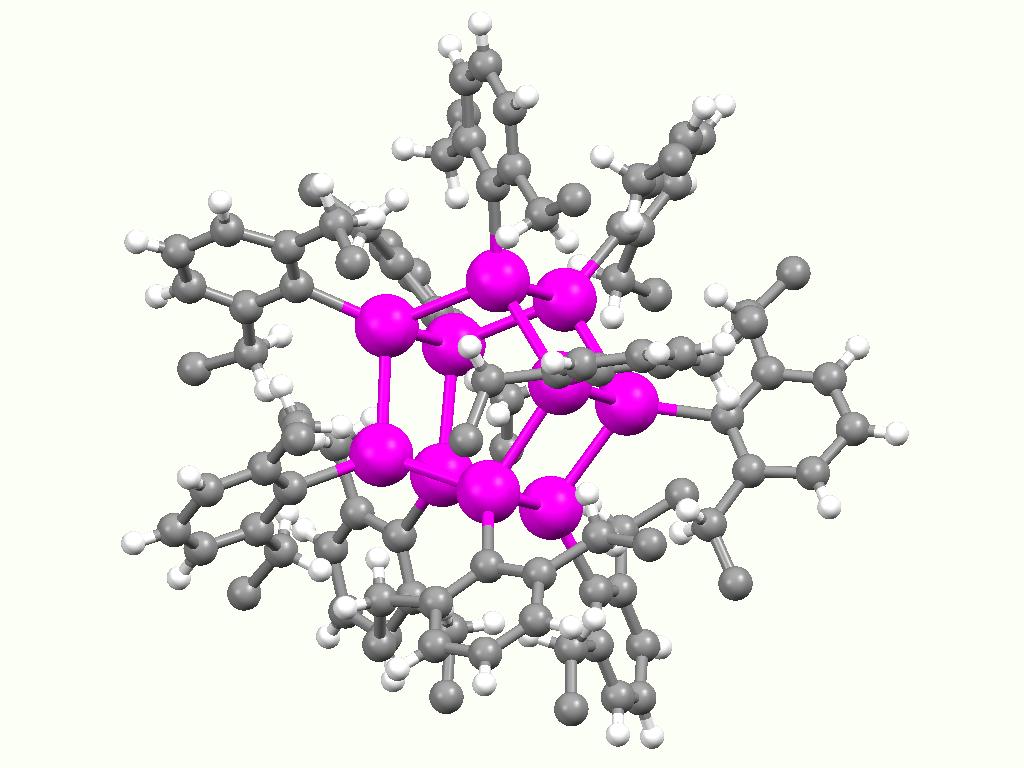
Triphenyltin hydroxide is an organotin compound with formula Sn(C6H5)3OH. Triphenyltin hydroxide is used as a fungicide for potatoes, sugar beets, and pecans. It was first registered for use as a pesticide in the United States in 1971. Structure While triphenyltin hydroxide is often depicted as a monomer, it crystallizes as a polymer A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic a ... with a bridging hydroxide groups. The Sn-O distances are 2.18 and 2.250 Å. Many organotin compounds engage in similar aggregation equilibria. References Triphenyltin compounds Fungicides {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organotin Compound
Organotin compounds or stannanes are chemical compounds based on tin with hydrocarbon substituents. Organotin chemistry is part of the wider field of organometallic chemistry. The first organotin compound was diethyltin diiodide (), discovered by Edward Frankland in 1849. The area grew rapidly in the 1900s, especially after the discovery of the Grignard reagents, which are useful for producing Sn–C bonds. The area remains rich with many applications in industry and continuing activity in the research laboratory. Structure Organotin compounds are generally classified according to their oxidation states. Tin(IV) compounds are much more common and more useful. Organic derivatives of tin(IV) The tetraorgano derivatives are invariably tetrahedral. Compounds of the type SnRR'R''R have been resolved into individual enantiomers. Organotin halides Organotin chlorides have the formula for values of ''n'' up to 3. Bromides, iodides, and fluorides are also known but less important. These ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fungicide
Fungicides are biocidal chemical compounds or biological organisms used to kill parasitic fungi or their spores. A fungistatic inhibits their growth. Fungi can cause serious damage in agriculture, resulting in critical losses of yield, quality, and profit. Fungicides are used both in agriculture and to fight fungal infections in animals. Chemicals used to control oomycetes, which are not fungi, are also referred to as fungicides, as oomycetes use the same mechanisms as fungi to infect plants. Fungicides can either be contact, translaminar or systemic. Contact fungicides are not taken up into the plant tissue and protect only the plant where the spray is deposited. Translaminar fungicides redistribute the fungicide from the upper, sprayed leaf surface to the lower, unsprayed surface. Systemic fungicides are taken up and redistributed through the xylem vessels. Few fungicides move to all parts of a plant. Some are locally systemic, and some move upwardly. Most fungicides that can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pesticide
Pesticides are substances that are meant to control pests. This includes herbicide, insecticide, nematicide, molluscicide, piscicide, avicide, rodenticide, bactericide, insect repellent, animal repellent, microbicide, fungicide, and lampricide. The most common of these are herbicides which account for approximately 80% of all pesticide use. Most pesticides are intended to serve as plant protection products (also known as crop protection products), which in general, protect plants from weeds, fungi, or insects. As an example, the fungus ''Alternaria solani'' is used to combat the aquatic weed ''Salvinia''. In general, a pesticide is a chemical (such as carbamate) or biological agent (such as a virus, bacterium, or fungus) that deters, incapacitates, kills, or otherwise discourages pests. Target pests can include insects, plant pathogens, weeds, molluscs, birds, mammals, fish, nematodes (roundworms), and microbes that destroy property, cause nuisance, or spread disease, or a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bridging Ligand
In coordination chemistry, a bridging ligand is a ligand that connects two or more atoms, usually metal ions. The ligand may be atomic or polyatomic. Virtually all complex organic compounds can serve as bridging ligands, so the term is usually restricted to small ligands such as pseudohalides or to ligands that are specifically designed to link two metals. In naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by the Greek letter mu, μ, with a subscript number denoting the number of metals bound to the bridging ligand. μ2 is often denoted simply as μ. When describing coordination complexes care should be taken not to confuse μ with η ('eta'), which relates to hapticity. Ligands that are not bridging are called terminal ligands. List of bridging ligands Virtually all ligands are known to bridge, with the exception of amines and ammonia. Common bridging ligands include most of the common anions. Many simple organic ligands form str ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |