|
Tonicity
In chemical biology, tonicity is a measure of the effective osmotic pressure gradient; the water potential of two solutions separated by a partially-permeable cell membrane. Tonicity depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic flux. It is commonly used when describing the swelling-versus-shrinking response of cells immersed in an external solution. Unlike osmotic pressure, tonicity is influenced only by solutes that cannot cross the membrane, as only these exert an effective osmotic pressure. Solutes able to freely cross the membrane do not affect tonicity because they will always equilibrate with equal concentrations on both sides of the membrane without net solvent movement. It is also a factor affecting imbibition. There are three classifications of tonicity that one solution can have relative to another: ''hypertonic'', ''hypotonic'', and ''isotonic''.A hypotonic s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmotic Pressure
Osmotic pressure is the minimum pressure which needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis. Potential osmotic pressure is the maximum osmotic pressure that could develop in a solution if it were separated from its pure solvent by a semipermeable membrane. Osmosis occurs when two solutions containing different concentrations of solute are separated by a selectively permeable membrane. Solvent molecules pass preferentially through the membrane from the low-concentration solution to the solution with higher solute concentration. The transfer of solvent molecules will continue until equilibrium is attained. Theory and measurement Jacobus van 't Hoff found a quantitative relationship between osmotic pressure and solute concentration, expressed in the following equation: :\Pi = icRT where \Pi is osmotic p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmole (unit)
Osmotic concentration, formerly known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L). The osmolarity of a solution is usually expressed as Osm/L (pronounced "osmolar"), in the same way that the molarity of a solution is expressed as "M" (pronounced "molar"). Whereas molarity measures the number of moles of solute per unit volume of solution, osmolarity measures the number of ''osmoles of solute particles'' per unit volume of solution. This value allows the measurement of the osmotic pressure of a solution and the determination of how the solvent will diffuse across a semipermeable membrane (osmosis) separating two solutions of different osmotic concentration. Unit The unit of osmotic concentration is the osmole. This is a non- SI unit of measurement that defines the number of moles of solute that contribute to the osmotic pressure of a solution. A milliosmole (mOsm) is 1/1,000 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dilute Solution
In chemistry, a solution is a special type of homogeneous mixture composed of two or more substances. In such a mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. These surrounded solute particles then move away from the solid solute and out into the solution. The mixing process of a solution happens at a scale where the effects of chemical polarity are involved, resulting in interactions that are specific to solvation. The solution usually has the state of the solvent when the solvent is the larger fraction of the mixture, as is commonly the case. One important parameter of a solution is the concentration, which is a measure of the amount of solute in a given amount of solution or solvent. The term "aqueous solution" is used when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blausen 0685 OsmoticFlow Isotonic
Blausen Medical Communications, Inc. is the creator and owner of a library of two- and three-dimensional medical and scientific images and animations, a developer of information technology allowing access to that content, and a business focused on licensing and distributing the content. It was founded by Bruce Blausen in Houston, Texas, in 1991, and is privately held. Background Blausen Medical Communications, Inc. (BMC) is a privately held company founded by Bruce Blausen in Houston, Texas in 1991. BMC created and owns a library of medical and scientific images and animations, and has developed information technology tools allowing access to the library; as well, it licenses and otherwise works to distribute the content. As of this date, BMC's animation library comprised approximately 1,500 animations and over 27,000 two- and three-dimensional images designed for point-of-care patient education, which could be accessed by consumers or professional caregivers (primarily via h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuole
A vacuole () is a membrane-bound organelle which is present in plant and fungal cells and some protist, animal, and bacterial cells. Vacuoles are essentially enclosed compartments which are filled with water containing inorganic and organic molecules including enzymes in solution, though in certain cases they may contain solids which have been engulfed. Vacuoles are formed by the fusion of multiple membrane vesicles and are effectively just larger forms of these. The organelle has no basic shape or size; its structure varies according to the requirements of the cell. Discovery Contractile vacuoles ("stars") were first observed by Spallanzani (1776) in protozoa, although mistaken for respiratory organs. Dujardin (1841) named these "stars" as ''vacuoles''. In 1842, Schleiden applied the term for plant cells, to distinguish the structure with cell sap from the rest of the protoplasm. In 1885, de Vries named the vacuole membrane as tonoplast. Function The function and signifi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pincushion
A pincushion (or pin cushion) is a small, stuffed cushion, typically across, which is used in sewing to store pins or needles with their heads protruding to take hold of them easily, collect them, and keep them organized. Pincushions are typically filled tightly with stuffing to hold pins rigidly in place. Magnetic pin cushions are also sometimes used; though technically they are not "cushions", they serve the same basic function of holding pins neatly. History The recorded origins of pincushions date back to the Middle Ages of Europe. In the English language, they became known by many names: "pimpilowes, pimpilos, pimplos, pimploes, pin-pillows, pin-poppets". In 1376, Jehanne de Mesnil was bequeathed a silver pin case in a French text called ''Testament of Advice'' written by a woman known as La Monteure, from Rouen. Other references to pin cases during the Medieval era exist. By the 16th century, these were supplanted by references to "pyn pillows". Some examples from variou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plasmodesmata
Plasmodesmata (singular: plasmodesma) are microscopic channels which traverse the cell walls of plant cells and some algal cells, enabling transport and communication between them. Plasmodesmata evolved independently in several lineages, and species that have these structures include members of the Charophyceae, Charales, Coleochaetales and Phaeophyceae (which are all algae), as well as all embryophytes, better known as land plants. Unlike animal cells, almost every plant cell is surrounded by a polysaccharide cell wall. Neighbouring plant cells are therefore separated by a pair of cell walls and the intervening middle lamella, forming an extracellular domain known as the apoplast. Although cell walls are permeable to small soluble proteins and other solutes, plasmodesmata enable direct, regulated, symplastic transport of substances between cells. There are two forms of plasmodesmata: primary plasmodesmata, which are formed during cell division, and secondary plasmodesmata, which c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Wall
A cell wall is a structural layer surrounding some types of cells, just outside the cell membrane. It can be tough, flexible, and sometimes rigid. It provides the cell with both structural support and protection, and also acts as a filtering mechanism. Cell walls are absent in many eukaryotes, including animals, but are present in some other ones like fungi, algae and plants, and in most prokaryotes (except mollicute bacteria). A major function is to act as pressure vessels, preventing over-expansion of the cell when water enters. The composition of cell walls varies between taxonomic group and species and may depend on cell type and developmental stage. The primary cell wall of land plants is composed of the polysaccharides cellulose, hemicelluloses and pectin. Often, other polymers such as lignin, suberin or cutin are anchored to or embedded in plant cell walls. Algae possess cell walls made of glycoproteins and polysaccharides such as carrageenan and agar that are absent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
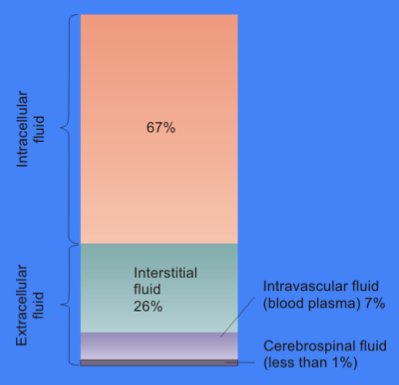
Cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells (intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondrion into many compartments. In the eukaryotic cell, the cytosol is surrounded by the cell membrane and is part of the cytoplasm, which also comprises the mitochondria, plastids, and other organelles (but not their internal fluids and structures); the cell nucleus is separate. The cytosol is thus a liquid matrix around the organelles. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others take place within organelles. The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and prope ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cell Membrane
The cell membrane (also known as the plasma membrane (PM) or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of all cells from the outside environment (the extracellular space). The cell membrane consists of a lipid bilayer, made up of two layers of phospholipids with cholesterols (a lipid component) interspersed between them, maintaining appropriate membrane fluidity at various temperatures. The membrane also contains membrane proteins, including integral proteins that span the membrane and serve as membrane transporters, and peripheral proteins that loosely attach to the outer (peripheral) side of the cell membrane, acting as enzymes to facilitate interaction with the cell's environment. Glycolipids embedded in the outer lipid layer serve a similar purpose. The cell membrane controls the movement of substances in and out of cells and organelles, being selectively permeable to ions a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Red Blood
''Red Blood'' is a 1925 American silent Western film directed by J.P. McGowan and starring Al Hoxie, Lew Meehan and Eddie Barry.Langman, p. 357 Cast * Al Hoxie as Buck Marsden * Marjorie Warfield as Edith Custer * Lew Meehan James Lew Meehan (September 7, 1890 – August 10, 1951) was an American film actor. Meehan appeared in more than 200 films between 1921 and 1947. He was often the main villain in silent Westerns, but in sound films he was usually an "anon ... as Dick 'Ace High' Willis * Eddie Barry as Donald Custer * J.P. McGowan as Eagle Custer * Frances Kellogg as Carlotta * Walter Patterson as Sodapop * Lem Sowards as 'Broom' References Bibliography * Langman, Larry. ''A Guide to Silent Westerns''. Greenwood Publishing Group, 1992. * McGowan, John J. ''J.P. McGowan: Biography of a Hollywood Pioneer''. McFarland, 2005. External links * 1925 films 1925 Western (genre) films American black-and-white films Films directed by J. P. McGowan Sil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imbibition
Imbibition is a special type of diffusion that takes place when liquid is absorbed by solids-colloids causing an increase in volume. Water surface potential movement takes place along a concentration gradient; some dry materials absorb water. A gradient between the absorbent and the liquid is essential for imbibition. For a substance to imbibe a liquid, there must first be some attraction between them. Imbibition occurs when a wetting fluid displaces a non-wetting fluid, the opposite of drainage in which a non-wetting phase displaces the wetting fluid. The two processes are governed by different mechanisms. Imbibition is also a type of diffusion since water movement is along the concentration gradient. The seeds and other such materials have almost no water hence they absorb water easily. Water potential gradient between the absorbent and liquid imbibed is essential for imbibition. Examples One example of imbibition in nature is the absorption of water by hydrophilic colloids. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |