|
Tilidine
Tilidine, or tilidate (brand names: Tilidin, Valoron, Tetova and Valtran) is a synthetic opioid painkiller, used mainly in Belgium, Bulgaria, Germany, Luxembourg, South Africa and Switzerland for the treatment of moderate to severe pain, both acute and chronic. Its onset of pain relief after oral administration is about 10–15 minutes and peak relief from pain occurs about 25–50 minutes after oral administration. Medical uses Tilidine is used in the form of hydrochloride or phosphate salt. In Germany, tilidine is available in a fixed combination with naloxone for oral administration (Valoron N and generics); the mixture of naloxone is claimed to lower the abuse liability of the opioid analgesic. This is so that if people take the medication orally (which is the way they are meant to) the opioid blocker, naloxone, has minimal effects on them but if they inject it the naloxone becomes bioavailable and hence antagonises the effects of the tilidine producing withdrawal effects. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tilidine Synthesis
Tilidine, or tilidate (brand names: Tilidin, Valoron, Tetova and Valtran) is a synthetic opioid painkiller, used mainly in Belgium, Bulgaria, Germany, Luxembourg, South Africa and Switzerland for the treatment of moderate to severe pain, both acute and chronic. Its onset of pain relief after oral administration is about 10–15 minutes and peak relief from pain occurs about 25–50 minutes after oral administration. Medical uses Tilidine is used in the form of hydrochloride or phosphate salt. In Germany, tilidine is available in a fixed combination with naloxone for oral administration (Valoron N and generics); the mixture of naloxone is claimed to lower the abuse liability of the opioid analgesic. This is so that if people take the medication orally (which is the way they are meant to) the opioid blocker, naloxone, has minimal effects on them but if they inject it the naloxone becomes bioavailable and hence antagonises the effects of the tilidine producing withdrawal effects. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nortilidine
Nortilidine is the major active metabolite of tilidine. It is formed from tilidine by demethylation in the liver. The racemate has opioid analgesic effects roughly equivalent in potency to that of morphine. The (1R,2S) isomer has NMDA antagonist activity. The drug also acts as a dopamine reuptake inhibitor. The reversed-ester of nortilidine is also known, as is the corresponding analogue with the cyclohexene ring replaced by cyclopentane, which have almost identical properties to nortilidine. Use Nortilidine has been sold as a designer drug, first being identified in Poland in May 2020. See also * Desmetramadol, another opioid metabolite with additional (non-opioid) mechanisms of analgesia, which has also been sold as a designer drug * Tapentadol Tapentadol, brand names Nucynta among others, is a centrally acting opioid analgesic of the benzenoid class with a dual mode of action as an agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Analges ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bisnortilidine
Bisnortilidine is an opioid metabolite. It is formed from tilidine by demethylation Demethylation is the chemical process resulting in the removal of a methyl group (CH3) from a molecule. A common way of demethylation is the replacement of a methyl group by a hydrogen atom, resulting in a net loss of one carbon and two hydrogen ato ... in the liver. References {{organic-compound-stub Human metabolites Cyclohexenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid
Opioids are substances that act on opioid receptors to produce morphine-like effects. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, reversing opioid overdose, and suppressing cough. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are also frequently used non-medically for their euphoric effects or to prevent withdrawal. Opioids can cause death and have been used for executions in the United States. Side effects of opioids may include itchiness, sedation, nausea, respiratory depression, constipation, and euphoria. Long-term use can cause tolerance, meaning that increased doses are required to achieve the same effect, and physical dependence, meaning that abruptly discontinuing the drug leads to unpleasant withdrawal symptoms. The euphoria attracts recreational use, and frequent, escalating recreational use of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Analgesic
An analgesic drug, also called simply an analgesic (American English), analgaesic (British English), pain reliever, or painkiller, is any member of the group of drugs used to achieve relief from pain (that is, analgesia or pain management). It is typically used to induce cooperation with a medical procedure. Analgesics are conceptually distinct from anesthetics, which temporarily reduce, and in some instances eliminate, sensation, although analgesia and anesthesia are neurophysiologically overlapping and thus various drugs have both analgesic and anesthetic effects. Analgesic choice is also determined by the type of pain: For neuropathic pain, traditional analgesics are less effective, and there is often benefit from classes of drugs that are not normally considered analgesics, such as tricyclic antidepressants and anticonvulsants. Various analgesics, such as many NSAIDs, are available over the counter in most countries, whereas various others are prescription drugs owing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CYP3A4
Cytochrome P450 3A4 (abbreviated CYP3A4) () is an important enzyme in the body, mainly found in the liver and in the intestine. It oxidizes small foreign organic molecules (xenobiotics), such as toxins or drugs, so that they can be removed from the body. It is highly homologous to CYP3A5, another important CYP3A enzyme. While many drugs are deactivated by CYP3A4, there are also some drugs which are ''activated'' by the enzyme. Some substances, such as some drugs and furanocoumarins present in grapefruit juice, interfere with the action of CYP3A4. These substances will therefore either amplify or weaken the action of those drugs that are modified by CYP3A4. CYP3A4 is a member of the cytochrome P450 family of oxidizing enzymes. Several other members of this family are also involved in drug metabolism, but CYP3A4 is the most common and the most versatile one. Like all members of this family, it is a hemoprotein, i.e. a protein containing a heme group with an iron atom. In humans, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hemihydrate
In chemistry, a hemihydrate (or semihydrate) is a hydrate whose solid contains one molecule of water of crystallization per two other molecules, or per two unit cell In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessaril ...s. An example of this is or , which is the hemihydrate of . References Hydrates {{chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back-for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamine
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one proton. Dimethylamine is a weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia at elevated temperatures and high pressure: :2 CH3OH + NH3 → (CH3)2NH + 2 H2O Natural occurrence Dimethylamine is found quite widely distributed in animals and plants, and is present in many foods at the level of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Crotonaldehyde
Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the ''E''- and ''Z''-isomers, which differ with respect to the relative position of the methyl and formyl groups. The ''E''-isomer is more common (data given in Table is for the ''E''-isomer). This lachrymatory liquid is moderately soluble in water and miscible in organic solvents. As an unsaturated aldehyde, crotonaldehyde is a versatile intermediate in organic synthesis. It occurs in a variety of foodstuffs, e.g. soybean oils. Production and reactivity Crotonaldehyde is produced by the aldol condensation of acetaldehyde: :2 CH3CHO → CH3CH=CHCHO + H2O Crotonaldehyde is a multifunctional molecule that exhibits diverse reactivity. It is a prochiral dienophile. It is a Michael acceptor. Addition of methylmagnesium chloride produces 3-penten-2-ol. Polyurethane catalyst ''N'',''N'',''N''′,''N''′-tetramethyl-1,4-butanediamine (also known as NIAX TMBDA) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
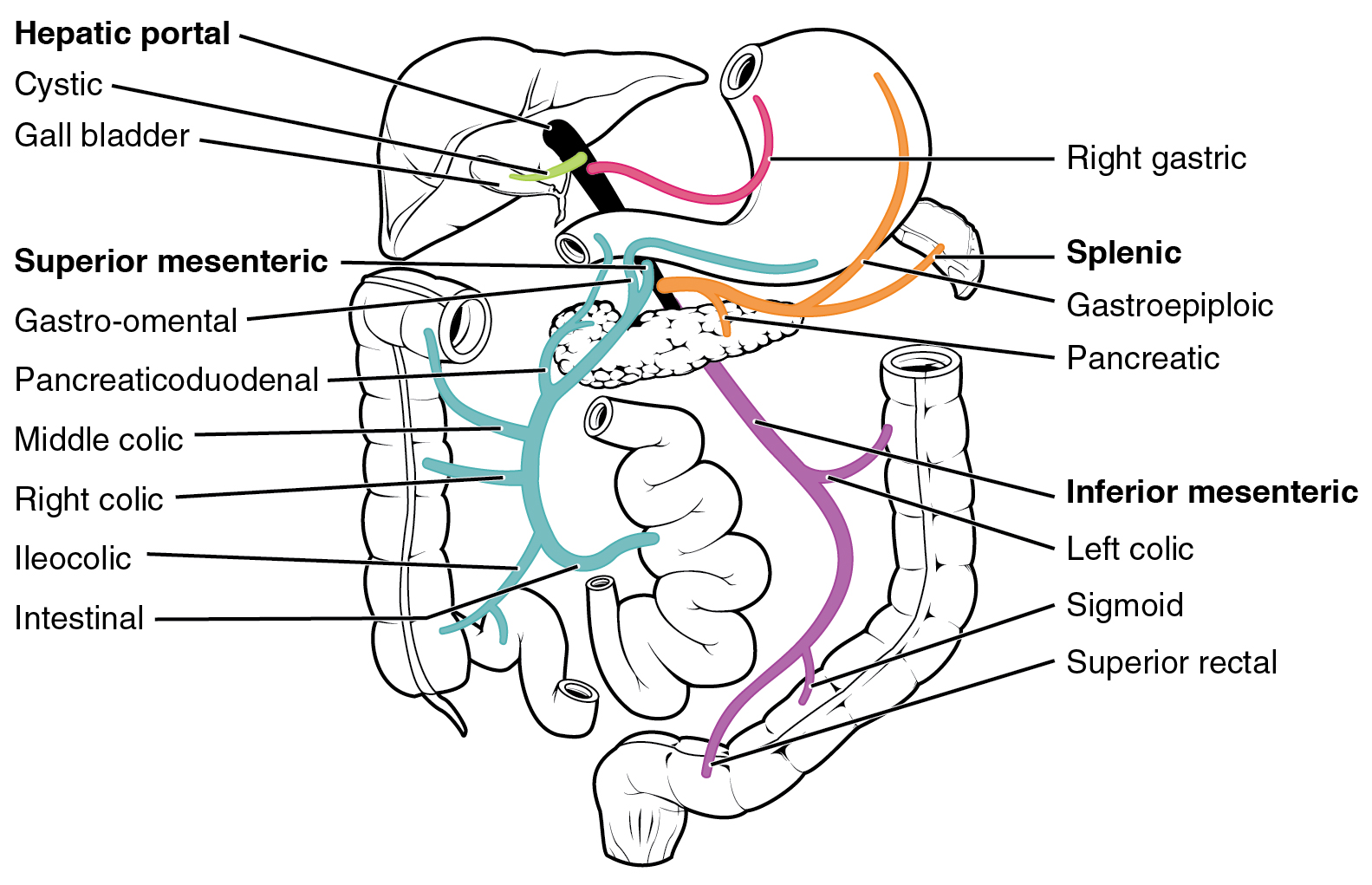
First Pass Effect
The first pass effect (also known as first-pass metabolism or presystemic metabolism) is a phenomenon of drug metabolism whereby the concentration of a drug, specifically when administered orally, is greatly reduced before it reaches the systemic circulation. It is the fraction of drug lost during the process of absorption which is generally related to the liver and gut wall. Notable drugs that experience a significant first-pass effect are buprenorphine, chlorpromazine, cimetidine, diazepam, ethanol (drinking alcohol), imipramine, insulin, lidocaine, midazolam, morphine, pethidine, propranolol, and tetrahydrocannabinol (THC). First pass metabolism may occur in the liver (for propranolol, lidocaine, clomethiazole, and NTG) or in the gut (for benzylpenicillin and insulin). After a drug is swallowed, it is absorbed by the digestive system and enters the hepatic portal system. It is carried through the portal vein into the liver before it reaches the rest of the body. The liver met ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |