|
Thorpe Reaction
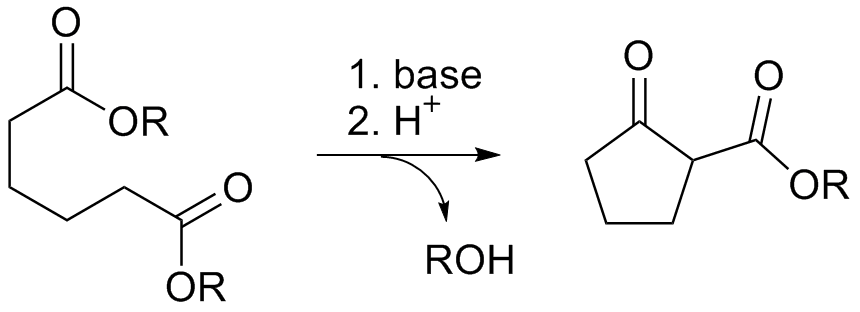
The Thorpe reaction is a chemical reaction described as a self-condensation of aliphatic nitriles catalyzed by base to form enamines. The reaction was discovered by Jocelyn Field Thorpe. Thorpe–Ziegler reaction The Thorpe–Ziegler reaction (named after Jocelyn Field Thorpe and Karl Ziegler), or Ziegler method, is the intramolecular modification with a dinitrile as a reactant and a cyclic ketone as the final reaction product after acidic hydrolysis. The reaction is conceptually related to the Dieckmann condensation. References {{Reflist External links * Thorpe-Ziegler reaction: ''4-Phosphorinanone, 1-phenyl-'' Organic Syntheses ''Organic Syntheses'' is a peer-reviewed scientific journal that was established in 1921. It publishes detailed and checked procedures for the synthesis of organic compounds. A unique feature of the review process is that all of the data and exp ..., Coll. Vol. 6, p. 932 (1988); Vol. 53, p. 98 (1973Link Carbon-carbon bond forming rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more products, which usually have properties different from the reactants. Reactions often consist of a sequence of individual sub-steps, the so-called elementary reactions, and the information on the precise course ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aliphatic
In organic chemistry, hydrocarbons ( compounds composed solely of carbon and hydrogen) are divided into two classes: aromatic compounds and aliphatic compounds (; G. ''aleiphar'', fat, oil). Aliphatic compounds can be saturated, like hexane, or unsaturated, like hexene and hexyne. Open-chain compounds, whether straight or branched, and which contain no rings of any type, are always aliphatic. Cyclic compounds can be aliphatic if they are not aromatic. Structure Aliphatic compounds can be saturated, joined by single bonds (alkanes), or unsaturated, with double bonds (alkenes) or triple bonds (alkynes). If other elements ( heteroatoms) are bound to the carbon chain, the most common being oxygen, nitrogen, sulfur, and chlorine, it is no longer a hydrocarbon, and therefore no longer an aliphatic compound. The least complex aliphatic compound is methane (CH4). Properties Most aliphatic compounds are flammable, allowing the use of hydrocarbons as fuel, such as me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including methyl cyanoacrylate, used in super glue, and nitrile rubber, a nitrile-containing polymer used in latex-free laboratory and medical gloves. Nitrile rubber is also widely used as automotive and other seals since it is resistant to fuels and oils. Organic compounds containing multiple nitrile groups are known as cyanocarbons. Inorganic compounds containing the group are not called nitriles, but cyanides instead. Though both nitriles and cyanides can be derived from cyanide salts, most nitriles are not nearly as toxic. Structure and basic properties The N−C−C geometry is linear in nitriles, reflecting the sp hybridization of the triply bonded carbon. The C−N distance is short at 1.16 Å, consistent with a triple bond. Nitr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Base (chemistry)
In chemistry, there are three definitions in common use of the word base, known as Arrhenius bases, Brønsted bases, and Lewis bases. All definitions agree that bases are substances that react with acids, as originally proposed by G.-F. Rouelle in the mid-18th century. In 1884, Svante Arrhenius proposed that a base is a substance which dissociates in aqueous solution to form Hydroxide ions OH−. These ions can react with hydrogen ions (H+ according to Arrhenius) from the dissociation of acids to form water in an acid–base reaction. A base was therefore a metal hydroxide such as NaOH or Ca(OH)2. Such aqueous hydroxide solutions were also described by certain characteristic properties. They are slippery to the touch, can taste bitter and change the color of pH indicators (e.g., turn red litmus paper blue). In water, by altering the autoionization equilibrium, bases yield solutions in which the hydrogen ion activity is lower than it is in pure water, i.e., the water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates. : The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the root ''amine''. This can be compared with enol, which is a functional group containing both alkene (''en''-) and alcohol (-''ol''). Enamines are considered to be nitrogen analogs of enols. If one of the nitrogen substituents is a hydrogen atom, H, it is the tautomeric form of an imine. This usually will rearrange to the imine; however there are several exceptions (such as aniline). The enamine-imine tautomerism may be considered analogous to the keto-enol tautomerism. In both cases, a hydrogen atom switches its location between the heteroatom (oxygen or nitrogen) and the second carbon atom. Enamines are both good nucleophiles and good bases. Their behavior as carbon-based nucleophiles is explained with reference to the following resonan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jocelyn Field Thorpe
Sir Jocelyn Field Thorpe FRS (1 December 1872 – 10 June 1940) was a British chemist who made major contributions to organic chemistry, including the Thorpe-Ingold effect and three named reactions. Early life and education Thorpe was born in Clapham, London on 1 December 1872, one of nine children and the sixth son, of Mr. and Mrs. W.G. Thorpe of the Middle Temple. He attended Worthing College, and then from 1888 - 1890 studied engineering at King's College, London. He then moved to the Royal College of Science from 1890 - 1892 to study chemistry. He earned his Ph.D. in organic chemistry under Karl von Auwers at Heidelberg University in 1895. In 1895 he joined Owens College, Manchester (this became part of the University of Manchester in 1904), starting as an assistant to W. H. Perkin Jr., becoming a lecturer in 1896 and senior lecturer in 1908. In that year he was elected FRS and was awarded a Sorby Fellowship by the Royal Society to study in Sheffield. Career and re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorpe Reaction Scheme
Thorpe is a variant of the Middle English word '' thorp'', meaning hamlet or small village. Thorpe may refer to: People * Thorpe (surname), including a list of people with the name Places England * Thorpe, Cumbria *Thorpe, Derbyshire * Thorpe, East Lindsey, Lincolnshire *Thorpe, East Riding of Yorkshire *Thorpe, North Yorkshire *Thorpe, Nottinghamshire * Thorpe, Surrey * Thorpe by Trusthorpe, Lincolnshire * Thorpe Hamlet, Norwich, Norfolk *Thorpe Hesley, South Yorkshire *Thorpe in Balne, South Yorkshire * Thorpe in the Fallows, Lincolnshire *Thorpe Latimer, Lincolnshire *Thorpe-le-Soken, Essex *Thorpe le Street, East Riding of Yorkshire *Thorpe on the Hill, Lincolnshire *Thorpe on the Hill, West Yorkshire * Thorpe St Andrew, Norfolk * Thorpe St Peter, Lincolnshire *Thorpe Tilney, Lincolnshire *Thorpe Waterville, Northamptonshire *Thorpe Willoughby, North Yorkshire Elsewhere *Thorpe, Missouri, a community in the United States See also *Littlethorpe, Leicestershire, England ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Karl Ziegler
Karl Waldemar Ziegler (26 November 1898 – 12 August 1973) was a German chemist who won the Nobel Prize in Chemistry in 1963, with Giulio Natta, for work on polymers. The Nobel Committee recognized his "excellent work on organometallic compounds hich..led to new polymerization reactions and ... paved the way for new and highly useful industrial processes". He is also known for his work involving free-radicals, many-membered rings, and organometallic compounds, as well as the development of Ziegler–Natta catalyst. One of many awards Ziegler received was the Werner von Siemens Ring in 1960 jointly with Otto Bayer and Walter Reppe, for expanding the scientific knowledge of and the technical development of new synthetic materials. Biography Early life and education Karl Ziegler was born on 26 November 1898 in Helsa near Kassel, Germany and was the second son of Karl Ziegler, a Lutheran minister, and Luise Rall Ziegler. He attended Kassel-Bettenhausen in elementary school. An in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Intramolecular Reaction
Intramolecular in chemistry describes a process or characteristic limited within the structure of a single molecule, a property or phenomenon limited to the extent of a single molecule. Examples * intramolecular hydride transfer (transfer of a hydride ion from one part to another within the same molecule) * intramolecular hydrogen bond (a hydrogen bond formed between two functional groups of the same molecule) *cyclization of ω-haloalkylamines and alcohols to form the corresponding saturated nitrogen and oxygen heterocycles, respectively (an SN2 reaction within the same molecule) In intramolecular organic reactions, two reaction sites are contained within a single molecule. This creates a very high effective concentration (resulting in high reaction rates), and, therefore, many intramolecular reactions that would not occur as an intermolecular reaction between two compounds take place. Examples of intramolecular reactions are the Smiles rearrangement, the Dieckmann condensatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considered re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dieckmann Condensation
The Dieckmann condensation is the intramolecular chemical reaction of diesters with base to give β-keto esters. It is named after the German chemist Walter Dieckmann (1869–1925). The equivalent intermolecular reaction is the Claisen condensation. : Reaction mechanism Deprotonation of an ester at the α-position generates an enolate ion which then undergoes a 5-exo-trig nucleophilic attack to give a cyclic enol. Protonation with a Brønsted-Lowry acid (H3O+ for example) re-forms the β-keto ester. : Due to the steric stability of five- and six-membered rings, these structures will preferentially be formed. 1,6 diesters will form five-membered cyclic β-keto esters, while 1,7 diesters will form six-membered β-keto esters. Further reading *Dieckmann, W. '' Ber.'' 1894, ''27'', 102 & 965 *Dieckmann, W. ''Ber.'' 1900, ''33'', 595 & 2670 *Dieckmann, W. ''Ann.'' 1901, ''317'', 51 & 93 See also * Claisen condensation The Claisen condensation is a carbon–carbon bond form ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thorpe Reaktion Mechanismus Schritt 1Version 5
Thorpe is a variant of the Middle English word '' thorp'', meaning hamlet or small village. Thorpe may refer to: People * Thorpe (surname), including a list of people with the name Places England * Thorpe, Cumbria *Thorpe, Derbyshire * Thorpe, East Lindsey, Lincolnshire *Thorpe, East Riding of Yorkshire *Thorpe, North Yorkshire *Thorpe, Nottinghamshire * Thorpe, Surrey * Thorpe by Trusthorpe, Lincolnshire * Thorpe Hamlet, Norwich, Norfolk *Thorpe Hesley, South Yorkshire *Thorpe in Balne, South Yorkshire * Thorpe in the Fallows, Lincolnshire *Thorpe Latimer, Lincolnshire *Thorpe-le-Soken, Essex *Thorpe le Street, East Riding of Yorkshire *Thorpe on the Hill, Lincolnshire *Thorpe on the Hill, West Yorkshire * Thorpe St Andrew, Norfolk * Thorpe St Peter, Lincolnshire *Thorpe Tilney, Lincolnshire *Thorpe Waterville, Northamptonshire *Thorpe Willoughby, North Yorkshire Elsewhere *Thorpe, Missouri, a community in the United States See also *Littlethorpe, Leicestershire, England ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |