|
Thioperamide
Thioperamide is a potency (pharmacology), potent Histamine H4 receptor, HRH4 antagonist and binding selectivity, selective histamine H3 receptor, HRH3 receptor antagonist, antagonist capable of crossing the blood–brain barrier. It was used by Jean-Charles Schwartz in his early experiments regarding the H3 receptor. Thioperamide was found to be an antagonist of histamine autoreceptors, which negatively regulate the release of histamine, and enhances the activity of histaminergic neurons by blocking autoreceptors, leading to greater release of histamine. Its action on H3 is thought to promote wakefulness and improve memory consolidation. See also * H3 antagonist, H3 receptor antagonist References H3 receptor antagonists Thioureas Piperidines Imidazoles Cyclohexyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H3 Antagonist
An H3 receptor antagonist is a classification of drugs used to block the action of histamine at the H3 receptor. Unlike the H1 and H2 receptors which have primarily peripheral actions, but cause sedation if they are blocked in the brain, H3 receptors are primarily found in the brain and are inhibitory autoreceptors located on histaminergic nerve terminals, which modulate the release of histamine. Histamine release in the brain triggers secondary release of excitatory neurotransmitters such as glutamate and acetylcholine via stimulation of H1 receptors in the cerebral cortex. Consequently, unlike the H1 antagonist antihistamines which are sedating, H3 antagonists have stimulant and nootropic effects, and are being researched as potential drugs for the treatment of neurodegenerative conditions such as Alzheimer's disease. Examples of selective H3 antagonists include clobenpropit, ABT-239, ciproxifan, conessine, A-349,821, betahistine, and pitolisant. History The histamine H3 re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine H4 Receptor
The histamine H4 receptor, like the other three histamine receptors, is a member of the G protein-coupled receptor superfamily that in humans is encoded by the ''HRH4'' gene. Discovery Unlike the histamine receptors discovered earlier, H4 was found in 2000 through a search of the human genomic DNA data base. Tissue distribution H4 is highly expressed in bone marrow and white blood cells and regulates neutrophil release from bone marrow and subsequent infiltration in the zymosan-induced pleurisy mouse model. It was also found that H4R exhibits a uniform expression pattern in the human oral epithelium. Function The Histamine H4 receptor has been shown to be involved in mediating eosinophil shape change and mast cell chemotaxis. This occurs via the βγ subunit acting at phospholipase C to cause actin polymerization and eventually chemotaxis. Structure The 3D structure of the H4 receptor has not been solved yet due to the difficulties of GPCR crystallization. Some attempts have ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Histamine H3 Receptor
Histamine H3 receptors are expressed in the central nervous system and to a lesser extent the peripheral nervous system, where they act as autoreceptors in presynaptic histaminergic neurons and control histamine turnover by feedback inhibition of histamine synthesis and release. The H3 receptor has also been shown to presynaptically inhibit the release of a number of other neurotransmitters (i.e. it acts as an inhibitory heteroreceptor) including, but probably not limited to dopamine, Gamma-aminobutyric acid, GABA, acetylcholine, noradrenaline, histamine and serotonin. The gene sequence for H3 receptors expresses only about 22% and 20% homology with both H1 and H2 receptors respectively. There is much interest in the histamine H3 receptor as a potential therapeutic target because of its involvement in the neuronal mechanism behind many cognitive disorders and especially its location in the central nervous system.Rapanelli, Maximiliano. “The Magnificent Two: Histamine and the H3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
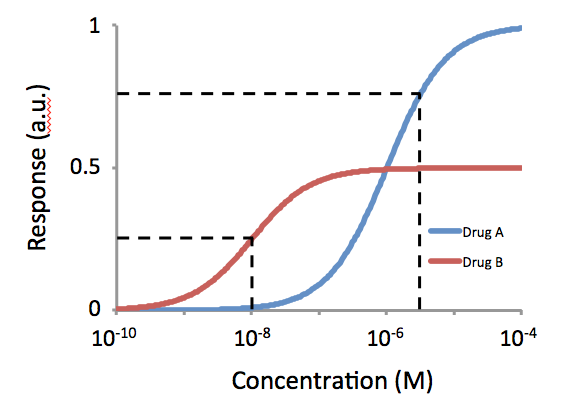
Potency (pharmacology)
In the field of pharmacology, potency is a measure of drug activity expressed in terms of the amount required to produce an effect of given intensity. A highly potent drug (e.g., fentanyl, alprazolam, risperidone, bumetanide, bisoprolol) evokes a given response at low concentrations, while a drug of lower potency (meperidine, diazepam, ziprasidone, furosemide, metoprolol) evokes the same response only at higher concentrations. Higher potency does not necessarily mean greater effectiveness or more side effects. The IUPHAR The International Union of Basic and Clinical Pharmacology (IUPHAR) is a voluntary, non-profit association representing the interests of scientists in pharmacology-related fields to facilitate ''Better Medicines through Global Education and Resear ... has stated that 'potency' is ''"an imprecise term that should always be further defined"'', for instance as EC_, IC_, ED_, LD_ and so on. See also * Reaction inhibitor § Potency References Further readin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Binding Selectivity
Binding selectivity is defined with respect to the binding of ligands to a substrate forming a complex. Binding selectivity describes how a ligand may bind more preferentially to one receptor than another. A selectivity coefficient is the equilibrium constant for the reaction of displacement by one ligand of another ligand in a complex with the substrate. Binding selectivity is of major importance in biochemistry and in chemical separation processes. Selectivity coefficient The concept of selectivity is used to quantify the extent to which one chemical substance, A, binds each of two other chemical substances, B and C. The simplest case is where the complexes formed have 1:1 stoichiometry. Then, the two interactions may be characterized by equilibrium constants ''K''AB and ''K''AC.The constant used here are ''association'' constants. ''Dissociation'' constants are used in some contexts. A dissociation constant is the reciprocal of an association constant. : + B AB; \mathit K_ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Receptor Antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of receptor proteins.Pharmacology Guide: In vitro pharmacology: concentration-response curves " '' GlaxoWellcome.'' Retrieved on December 6, 2007. They are sometimes called blockers; examples include alpha blockers, |
Blood–brain Barrier
The blood–brain barrier (BBB) is a highly selective semipermeable membrane, semipermeable border of endothelium, endothelial cells that prevents solutes in the circulating blood from ''non-selectively'' crossing into the extracellular fluid of the central nervous system where neurons reside. The blood–brain barrier is formed by endothelial cells of the Capillary, capillary wall, astrocyte end-feet ensheathing the capillary, and pericytes embedded in the capillary basement membrane. This system allows the passage of some small molecules by passive transport, passive diffusion, as well as the selective and active transport of various nutrients, ions, organic anions, and macromolecules such as glucose and amino acids that are crucial to neural function. The blood–brain barrier restricts the passage of pathogens, the diffusion of solutes in the blood, and Molecular mass, large or Hydrophile, hydrophilic molecules into the cerebrospinal fluid, while allowing the diffusion of Hydr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jean-Charles Schwartz
Jean-Charles Schwartz, born on May 28, 1936, in Paris, is a French neurobiologist, pharmacist and researcher. Husband of Ketty Schwartz, née Gersen (1937-2007) and father of Olivier, Marc and Emmanuelle. He is a member of the Academy of Sciences.. He developed pitolisant, the first clinically approved antagonist for H3 receptors. Biography Jean-Charles Schwartz holds a doctorate in pharmacy (Paris, 1960), a doctorate in science (Paris, 1965) and a diploma from the Institut de Pharmacodynamie et Pharmacotechnie (Paris, 1961). He was Professor of Physiology at the Faculties of Pharmacy of the Universities of Haute Normandie and Paris 5-René Descartes (1968-2001) and Professor of Neuropharmacology at the Institut Universitaire de France (1990-2000). He was a pharmacy internist (1958-1963), assistant at the Central Hospital Pharmacy (1964-1968) and then Chief Pharmacist at the Assistance Public-Hôpitaux de Paris (1968-1999). He headed the "Neurobiology and Pharmacology" and then ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Autoreceptor
An autoreceptor is a type of receptor located in the membranes of nerve cells. It serves as part of a negative feedback loop in signal transduction. It is only sensitive to the neurotransmitters or hormones released by the neuron on which the autoreceptor sits. Similarly, a heteroreceptor is sensitive to neurotransmitters and hormones that are not released by the cell on which it sits. A given receptor can act as either an autoreceptor or a heteroreceptor, depending upon the type of transmitter released by the cell on which it is embedded. Autoreceptors may be located in any part of the cell membrane: in the dendrites, the cell body, the axon, or the axon terminals. Canonically, a presynaptic neuron releases a neurotransmitter across a synaptic cleft to be detected by the receptors on a postsynaptic neuron. Autoreceptors on the presynaptic neuron will also detect this neurotransmitter and often function to control internal cell processes, typically inhibiting further release or ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
H3 Receptor Antagonists
H3, H03 or H-3 may refer to: Entertainment * ''Happy Hustle High'', a manga series by Rie Takada, originally titled "H3 School!" * H3 (film), ''H3'' (film), a 2001 film about the 1981 Irish hunger strike * h3h3Productions, styled "[h3]", a satirical YouTube channel Science * Triatomic hydrogen (H3), an unstable molecule * Trihydrogen cation (H3+), one of the most abundant ions in the universe * Tritium (Hydrogen-3, or H-3), an isotope of hydrogen * ATC code H03 ''Thyroid therapy'', a subgroup of the Anatomical Therapeutic Chemical Classification System * British NVC community H3, a heath community of the British National Vegetation Classification system * Histamine H3 receptor, Histamine H3 receptor, a human gene * Histone H3, a component of DNA higher structure in eukaryotic cells * *h3, , one of the three laryngeals in the reconstructed Proto-Indo-European language * Hekla 3 eruption, a huge volcanic eruption around 1000 BC Computing * , the level-3 HTML element#heading ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioureas
In organic chemistry, thioureas are members of a family of organosulfur compounds with the formula and structure . The parent member of this class of compounds is thiourea (). The thiourea functional group has a planar core. Structure and bonding Thioureas have planar core. The bond distance is near 1.71 Å, which is 0.1 Å longer than in normal ketones (). The C–N bond distances are short. Thioureas occurs in two tautomeric forms. For the parent thiourea, the thione form predominates in aqueous solutions. The thiol form, known as an isothiourea, can be encountered in substituted compounds such as isothiouronium salts. : Synthesis ''N'',''N''′-unsubstituted thioureas can be prepared by treating the corresponding cyanamide with hydrogen sulfide or similar sulfide sources. Organic ammonium salts react with potassium thiocyanate as the source of the thiocarbonyl (). Alternatively, ''N'',''N''′-disubstituted thioureas can be prepared by coupling two amines ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidines
Piperidine is an organic compound with the molecular formula (CH2)5NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges (–CH2–) and one amine bridge (–NH–). It is a colorless liquid with an odor described as objectionable, and typical of amines. The name comes from the genus name ''Piper'', which is the Latin word for pepper. Although piperidine is a common organic compound, it is best known as a representative structure element within many pharmaceuticals and alkaloids, such as natural-occurring solenopsins. Production Piperidine was first reported in 1850 by the Scottish chemist Thomas Anderson and again, independently, in 1852 by the French chemist Auguste Cahours, who named it. Both of them obtained piperidine by reacting piperine with nitric acid. Industrially, piperidine is produced by the hydrogenation of pyridine, usually over a molybdenum disulfide catalyst: : C5H5N + 3 H2 → C5H10NH Pyridine can also be reduce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |