|
Thermoplastic Elastomer
Thermoplastic elastomers (TPE), sometimes referred to as thermoplastic rubbers, are a class of copolymers or a physical mix of polymers (usually a plastic and a rubber) that consist of materials with both thermoplastic and elastomeric properties. While most elastomers are thermosets, thermoplastics are in contrast relatively easy to use in manufacturing, for example, by injection moulding. Thermoplastic elastomers show advantages typical of both rubbery materials and plastic materials. The benefit of using thermoplastic elastomers is the ability to stretch to moderate elongations and return to its near original shape creating a longer life and better physical range than other materials. The principal difference between thermoset elastomers and thermoplastic elastomers is the type of cross-linking bond in their structures. In fact, crosslinking is a critical structural factor which imparts high elastic properties. Types There are six generic classes of commercial TPEs (desig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are sometimes called ''bipolymers''. Those obtained from three and four monomers are called ''terpolymers'' and ''quaterpolymers'', respectively. Copolymers can be characterized by a variety of techniques such as NMR spectroscopy and size-exclusion chromatography to determine the molecular size, weight, properties, and composition of the material. Commercial copolymers include acrylonitrile butadiene styrene (ABS), styrene/butadiene co-polymer (SBR), nitrile rubber, styrene-acrylonitrile, styrene-isoprene-styrene (SIS) and ethylene-vinyl acetate, all of which are formed by chain-growth polymerization. Another production mechanism is step-growth polymerization, which is used to produce the nylon-12/6/66 copolymer of nylon 12, nylon 6 and nylon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Noryl
The NORYL family of modified resins consists of amorphous blends of polyphenylene oxides (PPO) or polyphenylene ether (PPE) resins with polystyrene. They combine the inherent benefits of PPE resin (affordable high heat resistance, good electrical insulation properties, excellent hydrolytic stability and the ability to use non-halogen fire retardant packages), with excellent dimensional stability, good processability and low density. They were originally developed in 1966 by General Electric Plastics (now owned by SABIC). NORYL is a registered trademark of SABIC Innovative Plastics IP B.V. NORYL resins are a rare example of a homogeneous mixture of two polymers. Most polymers are incompatible with one another, so tend to produce separate phases when mixed. The two polymers compatibility in NORYL resins is due to the presence of a benzene ring in the repeat units of both chains. Properties The addition of polystyrene to PPE increases the glass transition temperature above 100 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
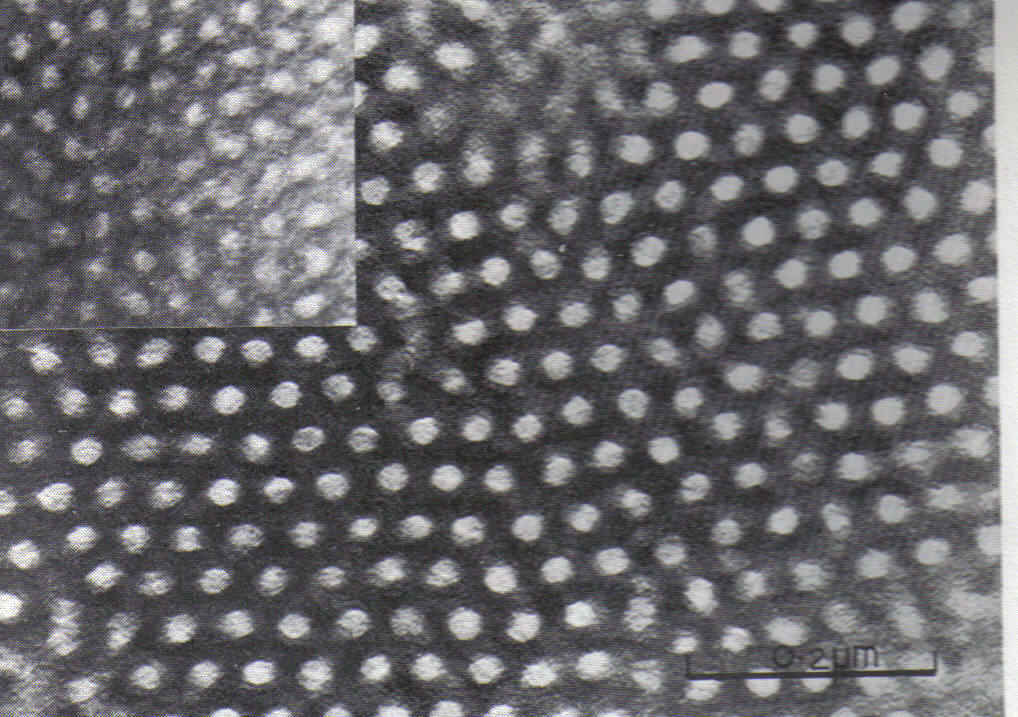
Microstructure
Microstructure is the very small scale structure of a material, defined as the structure of a prepared surface of material as revealed by an optical microscope above 25× magnification. The microstructure of a material (such as metals, polymers, ceramics or composites) can strongly influence physical properties such as strength, toughness, ductility, hardness, corrosion resistance, high/low temperature behaviour or wear resistance. These properties in turn govern the application of these materials in industrial practice. Microstructure at scales smaller than can be viewed with optical microscopes is often called nanostructure, while the structure in which individual atoms are arranged is known as crystal structure. The nanostructure of biological specimens is referred to as ultrastructure. A microstructure’s influence on the mechanical and physical properties of a material is primarily governed by the different defects present or absent of the structure. These defects can tak ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small-angle X-ray Scattering
Small-angle X-ray scattering (SAXS) is a small-angle scattering technique by which nanoscale density differences in a sample can be quantified. This means that it can determine nanoparticle size distributions, resolve the size and shape of (monodisperse) macromolecules, determine pore sizes, characteristic distances of partially ordered materials, and much more. This is achieved by analyzing the elastic scattering behaviour of X-rays when travelling through the material, recording their scattering at small angles (typically 0.1 – 10°, hence the "Small-angle" in its name). It belongs to the family of small-angle scattering (SAS) techniques along with small-angle neutron scattering, and is typically done using hard X-rays with a wavelength of 0.07 – 0.2 nm.. Depending on the angular range in which a clear scattering signal can be recorded, SAXS is capable of delivering structural information of dimensions between 1 and 100 nm, and of repeat distances in partially ordered s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and biochemistry, the distinction from ions is dropped and ''molecule'' is often used when referring to polyatomic ions. A molecule may be homonuclear, that is, it consists of atoms of one chemical element, e.g. two atoms in the oxygen molecule (O2); or it may be heteronuclear, a chemical compound composed of more than one element, e.g. water (two hydrogen atoms and one oxygen atom; H2O). In the kinetic theory of gases, the term ''molecule'' is often used for any gaseous particle regardless of its composition. This relaxes the requirement that a molecule contains two or more atoms, since the noble gases are individual atoms. Atoms and complexes connected by non-covalent interactions, such as hydrogen bonds or ionic bonds, are typically not consi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Living Polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer reactions are absent and the rate of chain initiation is also much larger than the rate of chain propagation. The result is that the polymer chains grow at a more constant rate than seen in traditional chain polymerization and their lengths remain very similar (i.e. they have a very low polydispersity index). Living polymerization is a popular method for synthesizing block copolymers since the polymer can be synthesized in stages, each stage containing a different monomer. Additional advantages are predetermined molar mass and control over end-groups. Living polymerization is desirable because it offers precision and control in macromolecular synthesis. This is important since many of the novel/useful properties of polymers result from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Osmium Tetroxide
Osmium tetroxide (also osmium(VIII) oxide) is the chemical compound with the formula OsO4. The compound is noteworthy for its many uses, despite its toxicity and the rarity of osmium. It also has a number of unusual properties, one being that the solid is volatile. The compound is colourless, but most samples appear yellow. This is most likely due to the presence of the impurity OsO2, which is yellow-brown in colour. In biology, its property of binding to lipids has made it a widely-used stain in electron microscopy. Physical properties Osmium(VIII) oxide forms monoclinic crystals. It has a characteristic acrid chlorine-like odor. The element name osmium is derived from ''osme'', Greek for ''odor''. OsO4 is volatile: it sublimes at room temperature. It is soluble in a wide range of organic solvents. It is also moderately soluble in water, with which it reacts reversibly to form osmic acid (see below). ''Pure'' osmium(VIII) oxide is probably colourless and it has been sugg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmission Electron Microscope
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a grid. An image is formed from the interaction of the electrons with the sample as the beam is transmitted through the specimen. The image is then magnified and focused onto an imaging device, such as a fluorescent screen, a layer of photographic film, or a sensor such as a scintillator attached to a charge-coupled device. Transmission electron microscopes are capable of imaging at a significantly higher resolution than light microscopes, owing to the smaller de Broglie wavelength of electrons. This enables the instrument to capture fine detail—even as small as a single column of atoms, which is thousands of times smaller than a resolvable object seen in a light microscope. Transmission electron microscopy is a major analytical method in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adhesive
Adhesive, also known as glue, cement, mucilage, or paste, is any non-metallic substance applied to one or both surfaces of two separate items that binds them together and resists their separation. The use of adhesives offers certain advantages over other binding techniques such as sewing, mechanical fastenings, or welding. These include the ability to bind different materials together, the more efficient distribution of stress across a joint, the cost-effectiveness of an easily mechanized process, and greater flexibility in design. Disadvantages of adhesive use include decreased stability at high temperatures, relative weakness in bonding large objects with a small bonding surface area, and greater difficulty in separating objects during testing. Adhesives are typically organized by the method of adhesion followed by ''reactive'' or ''non-reactive'', a term which refers to whether the adhesive chemically reacts in order to harden. Alternatively, they can be organized eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kraton (polymer)
Kraton is the trade name given to a number of high performance elastomers manufactured by Kraton Polymers, and used as synthetic replacements for rubber. Kraton polymers offers many of the properties of natural rubber, such as flexibility, high traction, and sealing abilities, but with increased resistance to heat, weathering, and chemicals. It was first made by the chemical division of the Shell Oil Company in the 1950s, under the technical leadership of Murray Luftglass and Norman R. Legge. Shell sold its Kraton polymers business to private equity firm Ripplewood Holdings in March 2001. Properties Kraton polymers are styrenic block copolymer (SBC) consisting of polystyrene blocks and rubber blocks. The rubber blocks consist of polybutadiene, polyisoprene or their hydrogenated equivalents. The tri-block with polystyrene blocks at both extremities linked together by a rubber block is the most important polymer structure observed in SBC. If the rubber block consist of polybutadie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |