|
Telluride (chemistry)
The telluride ion is the anion Te2− and its derivatives. It is analogous to the other chalcogenide anions, the lighter O2−, S2−, and Se2−, and the heavier Po2−. In principle, Te2− is formed by the two-e− reduction of tellurium. The redox potential is −1.14 V. :Te(s) + 2 e− ↔ Te2− Although solutions of the telluride dianion have not been reported, soluble salts of bitelluride (TeH−) are known. Organic tellurides ''Tellurides'' also describe a class of organotellurium compounds formally derived from Te2−. An illustrative member is dimethyl telluride, which results from the methylation of telluride salts: :2 CH3I + Na2Te → (CH3)2Te + 2 NaI Dimethyl telluride is formed by the body when tellurium is ingested. Such compounds are often called telluroethers because they are structurally related to ethers with tellurium replacing oxygen, although the length of the C–Te bond is much longer than a C–O bond. C–Te–C angles tend to be closer to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Telluride
Hydrogen telluride is the inorganic compound with the formula H2 Te. A hydrogen chalcogenide and the simplest hydride of tellurium, it is a colorless gas. Although unstable in ambient air, the gas can exist at very low concentrations long enough to be readily detected by the odour of rotting garlic at extremely low concentrations; or by the revolting odour of rotting leeks at somewhat higher concentrations. Most compounds with Te–H bonds (tellurols) are unstable with respect to loss of H2. H2Te is chemically and structurally similar to hydrogen selenide, both are acidic. The H–Te–H angle is about 90°. Volatile tellurium compounds often have unpleasant odours, reminiscent of decayed leeks or garlic.Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. . Synthesis Electrolytic methods have been developed.F. Fehér, "Hydrogen Telluride" in Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic P ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and wat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
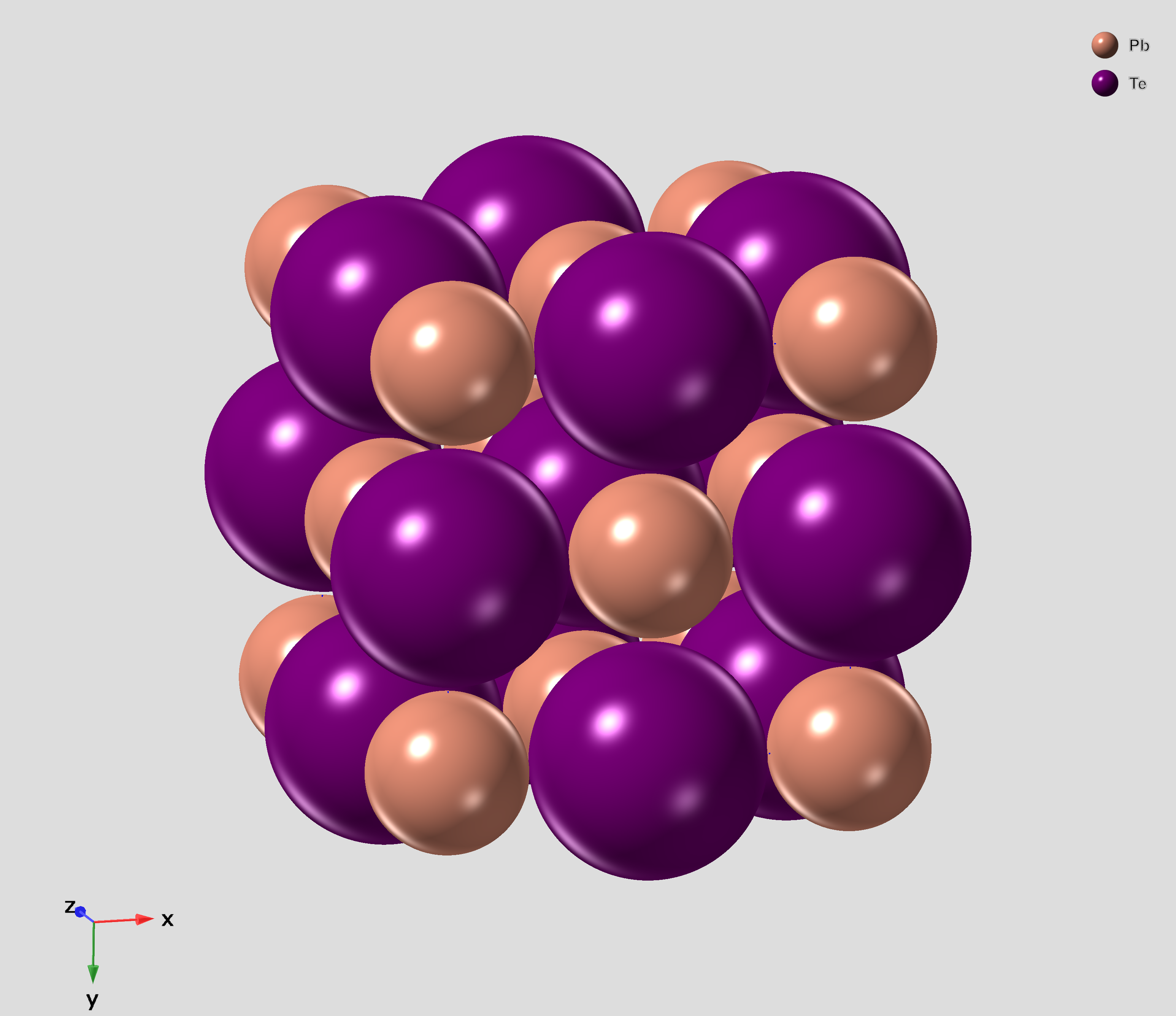
Lead Telluride
Lead telluride is a compound of lead and tellurium (PbTe). It crystallizes in the NaCl crystal structure with Pb atoms occupying the cation and Te forming the anionic lattice. It is a narrow gap semiconductor with a band gap of 0.32 eV. It occurs naturally as the mineral altaite. Properties * Dielectric constant ~1000. * Electron Effective mass ~ 0.01 ''m''e * Hole mobility, μp = 600 cm2 V−1 s−1 (0 K); 4000 cm2 V−1 s−1 (300 K) Applications PbTe has proven to be a very important intermediate thermoelectric material. The performance of thermoelectric materials can be evaluated by the figure of merit, ZT=S^2\sigma T/\kappa, in which S is the Seebeck coefficient, \sigma is the electrical conductivity and \kappa is the thermal conductivity. In order to improve the thermoelectric performance of materials, the power factor (S^2\sigma) needs to be maximized and the thermal conductivity needs to be minimized. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bismuth Telluride
Bismuth telluride (Bi2Te3) is a gray powder that is a compound of bismuth and tellurium also known as bismuth(III) telluride. It is a semiconductor, which, when alloyed with antimony or selenium, is an efficient thermoelectric material for refrigeration or portable power generation. Bi2Te3 is a topological insulator, and thus exhibits thickness-dependent physical properties. Properties as a thermoelectric material Bismuth telluride is a narrow-gap layered semiconductor with a trigonal unit cell. The valence and conduction band structure can be described as a many-ellipsoidal model with 6 constant-energy ellipsoids that are centered on the reflection planes. Bi2Te3 cleaves easily along the trigonal axis due to Van der Waals bonding between neighboring tellurium atoms. Due to this, bismuth-telluride-based materials used for power generation or cooling applications must be polycrystalline. Furthermore, the Seebeck coefficient of bulk Bi2Te3 becomes compensated around room temperatur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photovoltaic
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially used for electricity generation and as photosensors. A photovoltaic system employs solar modules, each comprising a number of solar cells, which generate electrical power. PV installations may be ground-mounted, rooftop-mounted, wall-mounted or floating. The mount may be fixed or use a solar tracker to follow the sun across the sky. Photovoltaic technology helps to mitigate climate change because it emits much less carbon dioxide than fossil fuels. Solar PV has specific advantages as an energy source: once installed, its operation generates no pollution and no greenhouse gas emissions, it shows scalability in respect of power needs and silicon has large availability in the Earth's crust, although other materials required in PV sys ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadmium Telluride
Cadmium telluride (CdTe) is a stable crystalline compound formed from cadmium and tellurium. It is mainly used as the semiconducting material in cadmium telluride photovoltaics and an infrared optical window. It is usually sandwiched with cadmium sulfide to form a p–n junction solar PV cell. Applications CdTe is used to make thin film solar cells, accounting for about 8% of all solar cells installed in 2011. They are among the lowest-cost types of solar cell, although a comparison of total installed cost depends on installation size and many other factors, and has changed rapidly from year to year. The CdTe solar cell market is dominated by First Solar. In 2011, around 2 GWp of CdTe solar cells were produced; For more details and discussion see cadmium telluride photovoltaics. CdTe can be alloyed with mercury to make a versatile infrared detector material ( HgCdTe). CdTe alloyed with a small amount of zinc makes an excellent solid-state X-ray and gamma ray detector ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polytelluride
In chemistry Chemistry is the science, scientific study of the properties and behavior of matter. It is a natural science that covers the Chemical element, elements that make up matter to the chemical compound, compounds made of atoms, molecules and ions ..., a polytelluride usually refers to anions of the formula (Ten)2-. Many main group and transition metals form complexes with polytelluride anions. Preparation Conceptually, polytellurides are derived from polytelluranes H2Ten, but such neutral species are not known (even H2Te is labile). Instead, analogous to the preparation of many Zintl ions, polytellurides are produced by reduction of elemental Te with alkali metals. Such reactions can be conducted by heating a mixture of the solids or by dissolving Te metal in amine solvents of alkali metals. Once generated, these alkali metal polytellurides can be converted to lipophilic salts by treatment cryptand ligands or by ion exchange with quat salts. Structures Sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aurostibite
Aurostibite is an isometric gold antimonide mineral which is a member of the pyrite group. Aurostibite was discovered in 1952 and can be found in hydrothermal gold-quartz veins, in sulfur-deficient environments that contain other antimony minerals. The mineral can be found in Yellowknife in the Northwest Territories of Canada, and the Timiskaming District in Ontario, Canada. Antimonides are rare and are normally placed in the sulfide class by mineralogists. See also * List of minerals This is a list of minerals for which there are articles on Wikipedia. Minerals are distinguished by various chemical and physical properties. Differences in chemical composition and crystal structure distinguish the various ''species''. Within a m ... References Gold minerals Antimonide minerals Pyrite group Cubic minerals Minerals in space group 205 Minerals described in 1952 {{mineral-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sylvanite
Sylvanite or silver gold telluride, chemical formula , is the most common telluride of gold. Properties The gold:silver ratio varies from 3:1 to 1:1. It is a metallic mineral with a color that ranges from a steely gray to almost white. It is closely related to calaverite, which is more purely gold telluride with 3% silver. Sylvanite crystallizes in the monoclinic 2/m system. Crystals are rare and it is usually bladed or granular. It is very soft with a hardness of 1.5–2. It has a high relative density of 8–8.2. Sylvanite is photosensitive and can accumulate a dark tarnish if it is exposed to bright light for too long. Occurrence Sylvanite is found in Transylvania, from which its name is partially derived. It is also found and mined in Australia in the East Kalgoorlie district. In Canada it is found in the Kirkland Lake Gold District, Ontario and the Rouyn District, Quebec. In the United States it occurs in California and in Colorado where it was mined as part of the Crip ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Krennerite
Krennerite is an orthorhombic gold telluride mineral which can contain variable amounts of silver in the structure. The formula is AuTe2, but specimen with gold substituted by up to 24% with silver have been found ( u0.77Ag0.24e2). Both of the chemically similar gold-silver tellurides, calaverite and sylvanite, are in the monoclinic crystal system, whereas krennerite is orthorhombic. The color varies from silver-white to brass-yellow. It has a specific gravity of 8.62 and a hardness of 2.5. It occurs in high temperature, hydrothermal environments. Krennerite was discovered in 1878 near the village of Săcărâmb, Romania, and first described by the Hungarian mineralogist Joseph Krenner (1839–1920). See also *List of minerals *List of minerals named after people This is a list of minerals named after people. The chemical composition follows name. A * Abelsonite: C31H32N4Ni – American physicist Philip Hauge Abelson (1913–2004)alfred * Abswurmbachite: Cu2+Mn3+6 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calaverite
Calaverite, or gold telluride, is an uncommon telluride of gold, a metallic mineral with the chemical formula AuTe2, with approximately 3% of the gold replaced by silver. It was first discovered in Calaveras County, California in 1861, and was named for the county in 1868. The mineral often has a metallic luster, and its color may range from a silvery white to a brassy yellow. It is closely related to the gold-silver telluride mineral sylvanite, which, however, contains significantly more silver. Another AuTe2 mineral (but with a quite different crystal structure) is krennerite. Calaverite and sylvanite represent the major telluride ores of gold, although such ores are minor sources of gold in general. As a major gold mineral found in Western Australia, calaverite played a major role in the 1890s gold rushes in that area. Physical and chemical properties Calaverite occurs as monoclinic crystals, which do not possess cleavage planes. It has a specific gravity of 9.35 and a hard ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |