|
Technetium Hexafluoride
Technetium hexafluoride or technetium(VI) fluoride ( Tc F6) is a yellow inorganic compound with a low melting point. It was first identified in 1961. In this compound, technetium has an oxidation state of +6, the highest oxidation state found in the technetium halides. In this respect, technetium differs from rhenium, which forms a heptafluoride, ReF7. Technetium hexafluoride occurs as an impurity in uranium hexafluoride, as technetium is a fission product of uranium ( spontaneous fission in natural uranium, possible contamination from induced fission inside the reactor in reprocessed uranium). The fact that the boiling point of the hexafluorides of uranium and technetium are very close to each other presents a problem in using fluoride volatility in nuclear reprocessing. Preparation Technetium hexafluoride is prepared by heating technetium metal with an excess of F2 at 400 °C. :Tc + 3 → Description Technetium hexafluoride is a golden-yellow solid at room tem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Inorganic Chemistry (journal)
''Inorganic Chemistry'' is a biweekly peer-reviewed scientific journal published by the American Chemical Society since 1962. It covers research in all areas of inorganic chemistry. The current editor-in-chief is William B. Tolman (Washington University). Abstracting and indexing The journal is abstracted and indexed in: According to the ''Journal Citation Reports'', the journal has a 2021 impact factor of 5.436. See also * ''Organometallics ''Organometallics'' is a biweekly journal published by the American Chemical Society. Its area of focus is organometallic and organometalloid chemistry. This peer-reviewed journal has an impact factor of 3.837 as reported by the 2021 Journal Cit ...'' References External links * American Chemical Society academic journals Biweekly journals Publications established in 1962 English-language journals Inorganic chemistry journals {{chem-journal-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluoride Volatility
Fluoride volatility is the tendency of highly fluorinated molecules to vaporize at comparatively low temperatures. Heptafluorides, hexafluorides and pentafluorides have much lower boiling points than the lower- valence fluorides. Most difluorides and trifluorides have high boiling points, while most tetrafluorides and monofluorides fall in between. The term "fluoride volatility" is jargon used particularly in the context of separation of radionuclides. Volatility and valence Valences for the majority of elements are based on the highest known fluoride. Roughly, fluoride volatility can be used to remove elements with a valence of 5 or greater: uranium, neptunium, plutonium, metalloids (tellurium, antimony), nonmetals (selenium), halogens (iodine, bromine), and the middle transition metals (niobium, molybdenum, technetium, ruthenium, and possibly rhodium). This fraction includes the actinides most easily reusable as nuclear fuel in a thermal reactor, and the two long-lived f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bohr Magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum. The Bohr magneton, in SI units is defined as \mu_\mathrm = \frac , And in Gaussian CGS units: \mu_\mathrm = \frac , where * is the elementary charge, * is the reduced Planck constant, * is the electron rest mass, * is the speed of light. History The idea of elementary magnets is due to Walther Ritz (1907) and Pierre Weiss. Already before the Rutherford model of atomic structure, several theorists commented that the magneton should involve the Planck constant ''h''. By postulating that the ratio of electron kinetic energy to orbital frequency should be equal to ''h'', Richard Gans computed a value that was twice as large as the Bohr magneton in September 1911. At the First Solvay Conference in November that year, Paul Langevin obtained a e\hbar/(12m_\mathrm). Langevin assumed that the attracti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Moment
In electromagnetism, the magnetic moment is the magnetic strength and orientation of a magnet or other object that produces a magnetic field. Examples of objects that have magnetic moments include loops of electric current (such as electromagnets), permanent magnets, elementary particles (such as electrons), various molecules, and many astronomical objects (such as many planets, some moons, stars, etc). More precisely, the term ''magnetic moment'' normally refers to a system's magnetic dipole moment, the component of the magnetic moment that can be represented by an equivalent magnetic dipole: a magnetic north and south pole separated by a very small distance. The magnetic dipole component is sufficient for small enough magnets or for large enough distances. Higher-order terms (such as the magnetic quadrupole moment) may be needed in addition to the dipole moment for extended objects. The magnetic dipole moment of an object is readily defined in terms of the torque that the objec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bond Length
In molecular geometry, bond length or bond distance is defined as the average distance between nuclei of two bonded atoms in a molecule. It is a transferable property of a bond between atoms of fixed types, relatively independent of the rest of the molecule. Explanation Bond length is related to bond order: when more electrons participate in bond formation the bond is shorter. Bond length is also inversely related to bond strength and the bond dissociation energy: all other factors being equal, a stronger bond will be shorter. In a bond between two identical atoms, half the bond distance is equal to the covalent radius. Bond lengths are measured in the solid phase by means of X-ray diffraction, or approximated in the gas phase by microwave spectroscopy. A bond between a given pair of atoms may vary between different molecules. For example, the carbon to hydrogen bonds in methane are different from those in methyl chloride. It is however possible to make generalizations when ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Symmetry
A regular octahedron has 24 rotational (or orientation-preserving) symmetries, and 48 symmetries altogether. These include transformations that combine a reflection and a rotation. A cube has the same set of symmetries, since it is the polyhedron that is dual polyhedron, dual to an octahedron. The group of orientation-preserving symmetries is ''S''4, the symmetric group or the group of permutations of four objects, since there is exactly one such symmetry for each permutation of the four diagonals of the cube. Details Chiral and full (or achiral) octahedral symmetry are the Point groups in three dimensions, discrete point symmetries (or equivalently, List of spherical symmetry groups, symmetries on the sphere) with the largest symmetry groups compatible with translational symmetry. They are among the Crystal system#Overview of point groups by crystal system, crystallographic point groups of the cubic crystal system. As the hyperoctahedral group of dimension 3 the full oct ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octahedral Molecular Geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The octahedron has eight faces, hence the prefix ''octa''. The octahedron is one of the Platonic solids, although octahedral molecules typically have an atom in their centre and no bonds between the ligand atoms. A perfect octahedron belongs to the point group Oh. Examples of octahedral compounds are sulfur hexafluoride SF6 and molybdenum hexacarbonyl Mo(CO)6. The term "octahedral" is used somewhat loosely by chemists, focusing on the geometry of the bonds to the central atom and not considering differences among the ligands themselves. For example, , which is not octahedral in the mathematical sense due to the orientation of the bonds, is referred to as octahedral. The concept of octahedral coordination geometry was developed by Alfred Wern ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Angstrom
The angstromEntry "angstrom" in the Oxford online dictionary. Retrieved on 2019-03-02 from https://en.oxforddictionaries.com/definition/angstrom.Entry "angstrom" in the Merriam-Webster online dictionary. Retrieved on 2019-03-02 from https://www.merriam-webster.com/dictionary/angstrom. (, ; , ) or ångström is a metric unit of length equal to m; that is, one ten-billionth ( US) of a metre, a hundred-millionth of a centimetre,Entry "angstrom" in the Oxford English Dictionary, 2nd edition (1986). Retrieved on 2021-11-22 from https://www.oed.com/oed2/00008552. 0.1 nanometre, or 100 picometres. Its symbol is Å, a letter of the Swedish alphabet. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). The angstrom is often used in the natural sciences and technology to express sizes of atoms, molecules, microscopic biological structures, and lengths of chemical bonds, arrangement of atoms in crystals,Arturas Vailionis (2015):Geometry of Crystals Lect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthorhombic Crystal System
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic lattices result from stretching a cubic lattice along two of its orthogonal pairs by two different factors, resulting in a rectangular prism with a rectangular base (''a'' by ''b'') and height (''c''), such that ''a'', ''b'', and ''c'' are distinct. All three bases intersect at 90° angles, so the three lattice vectors remain mutually orthogonal. Bravais lattices There are four orthorhombic Bravais lattices: primitive orthorhombic, base-centered orthorhombic, body-centered orthorhombic, and face-centered orthorhombic. For the base-centered orthorhombic lattice, the primitive cell has the shape of a right rhombic prism;See , row oC, column Primitive, where the cell parameters are given as a1 = a2, α = β = 90° it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
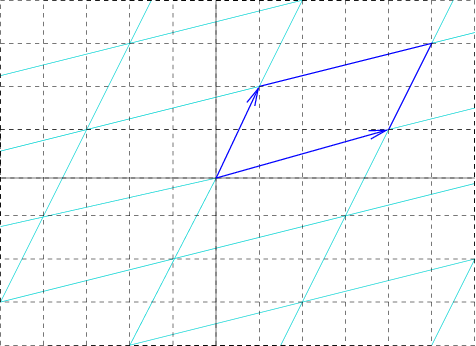
Unit Cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector, for example) does not necessarily have unit size, or even a particular size at all. Rather, the primitive cell is the closest analogy to a unit vector, since it has a determined size for a given lattice and is the basic building block from which larger cells are constructed. The concept is used particularly in describing crystal structure in two and three dimensions, though it makes sense in all dimensions. A lattice can be characterized by the geometry of its unit cell, which is a section of the tiling (a parallelogram or parallelepiped) that generates the whole tiling using only translations. There are two special cases of the unit cell: the primitive cell and the conventional cell. The primitive cell is a unit cell corresponding to a single lattice point, it is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formula Unit
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound. Examples include ionic and and covalent networks such as and C (as diamond or graphite).Steven S. Zumdahl; Susan A. Zumdahl (2000), ''Chemistry'' (5 ed.), Houghton Mifflin, pp. 470-6, Ionic compounds do not exist as individual molecules; a formula unit thus indicates the lowest reduced ratio of ions in the compound. In mineralogy, as minerals are almost exclusively either ionic or network solids, the formula unit is used. The number of formula units (Z) and the dimensions of the crystallographic axes are used in defining the unit cell In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lattice Constant
A lattice constant or lattice parameter is one of the physical dimensions and angles that determine the geometry of the unit cells in a crystal lattice, and is proportional to the distance between atoms in the crystal. A simple cubic crystal has only one lattice constant, the distance between atoms, but in general lattices in three dimensions have six lattice constants: the lengths ''a'', ''b'', and ''c'' of the three cell edges meeting at a vertex, and the angles ''α'', ''β'', and ''γ'' between those edges. The crystal lattice parameters ''a'', ''b'', and ''c'' have the dimension of length. The three numbers represent the size of the unit cell, that is, the distance from a given atom to an identical atom in the same position and orientation in a neighboring cell (except for very simple crystal structures, this will not necessarily be disance to the nearest neighbor). Their SI unit is the meter, and they are traditionally specified in angstroms (Å); an angstrom being 0.1 nanome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |