|
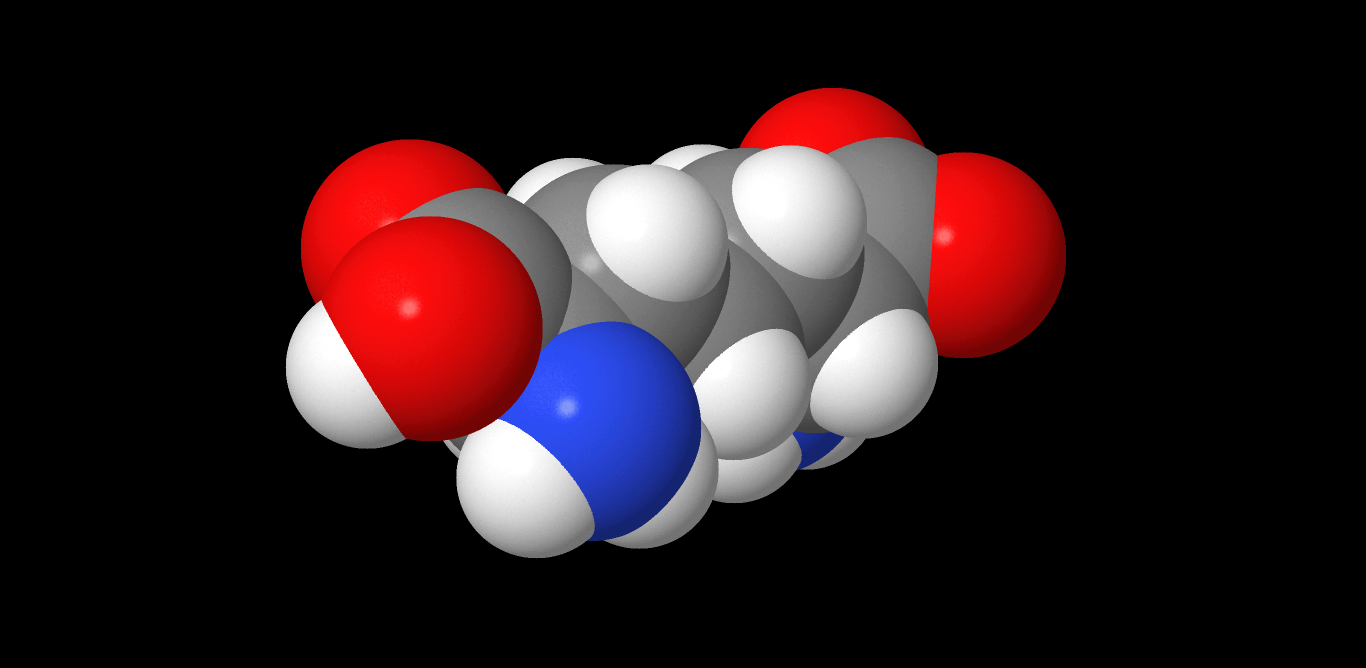
Tabtoxin
Tabtoxin, also known as wildfire toxin, is a simple monobactam phytotoxin produced by ''Pseudomonas syringae''. It is the precursor to the antibiotic tabtoxinine β-lactam (TBL). It is produced by: * Pseudomonas syringae pv. tabaci, the causal agent of the wildfire of tobacco. * P. syringae pv. coronafaciens * P. syringae pv. garcae * P. syringae BR2, causes a disease of bean (Phaseolus vulgaris) similar to tobacco wildfire. This organism is closely related to P. syringae pv. tabaci but cannot be classified in the pathovar tabaci because it is not pathogenic on tobacco. Tabtoxin is a dipeptide precursor to the biologically active form of TBL, differing by having an extra threonine attached by a peptide bond to the C terminus. Tabtoxin is required by BR2(R) for both chlorosis and lesion formation on bean. All mutations that affected tabtoxin production, whether spontaneous deletion or transposon induced, also affected lesion formation, and in all cases, restoration of tabtoxin produ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tabtoxinine β-lactam
Tabtoxinine β-lactam is a monobactam antibiotic derived from tabtoxin. Unlike other beta-lactam antibiotic, β-lactam antibiotics, which inhibit penicillin-binding proteins, tabtoxinine β-lactam inhibits glutamine synthetase. References Lactams Amino acid derivatives {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phytotoxin
Phytotoxins are substances that are poisonous or toxic to the growth of plants. Phytotoxic substances may result from human activity, as with herbicides, or they may be produced by plants, by microorganisms, or by naturally occurring chemical reactions. The term is also used to describe toxic chemicals produced by plants themselves, which function as defensive agents against their predators. Most examples pertaining to this definition of phytotoxin are members of various classes of specialised or secondary metabolites, including alkaloids, terpenes, and especially phenolics, though not all such compounds are toxic or serve defensive purposes. Phytotoxins may also be toxic to humans. Toxins produced by plants Alkaloids Alkaloids are derived from amino acids, and contain nitrogen. They are medically important by interfering with components of the nervous system affecting membrane transport, protein synthesis, and enzyme activities. They generally have a bitter taste. Alkaloids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monobactam
Monobactams are monocyclic and bacterially-produced β-lactam antibiotics. The β-lactam ring is not fused to another ring, in contrast to most other β-lactams. Monobactams are effective only against aerobic Gram-negative bacteria (e.g., ''Neisseria'', ''Pseudomonas''). Siderophore-conjugated monobactams show promise for the treatment of multi drug-resistant pathogens. Aztreonam is a commercially available monobactam antibiotic. Other examples of monobactams are tigemonam, nocardicin A, and tabtoxin. Adverse effects to monobactams can include skin rash and occasional abnormal liver functions. Monobactam antibiotics exhibit no IgE cross-reactivity reactions with penicillin but have shown some cross reactivity with cephalosporins, most notably ceftazidime Ceftazidime, sold under the brand name Fortaz among others, is a third-generation cephalosporin antibiotic useful for the treatment of a number of bacterial infections. Specifically it is used for joint infections, menin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bacterial Toxins
Microbial toxins are toxins produced by micro-organisms, including bacteria, fungi, protozoa, dinoflagellates, and viruses. Many microbial toxins promote infection and disease by directly damaging host tissues and by disabling the immune system. Endotoxins most commonly refer to the lipopolysaccharide (LPS) or lipooligosaccharide (LOS) that are in the outer plasma membrane of Gram-negative bacteria. The botulinum toxin, which is primarily produced by ''Clostridium botulinum'' and less frequently by other '' Clostridium'' species, is the most toxic substance known in the world. However, microbial toxins also have important uses in medical science and research. Currently, new methods of detecting bacterial toxins are being developed to better isolate and understand these toxin. Potential applications of toxin research include combating microbial virulence, the development of novel anticancer drugs and other medicines, and the use of toxins as tools in neurobiology and cellular biolo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid Derivatives
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. Aromatic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyltransferase
Methyltransferases are a large group of enzymes that all methylate their substrates but can be split into several subclasses based on their structural features. The most common class of methyltransferases is class I, all of which contain a Rossmann fold for binding ''S''-Adenosyl methionine (SAM). Class II methyltransferases contain a SET domain, which are exemplified by SET domain histone methyltransferases, and class III methyltransferases, which are membrane associated. Methyltransferases can also be grouped as different types utilizing different substrates in methyl transfer reactions. These types include protein methyltransferases, DNA/RNA methyltransferases, natural product methyltransferases, and non-SAM dependent methyltransferases. SAM is the classical methyl donor for methyltransferases, however, examples of other methyl donors are seen in nature. The general mechanism for methyl transfer is a SN2-like nucleophilic attack where the methionine sulfur serves as the leavi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diaminopimelate Decarboxylase
The enzyme diaminopimelate decarboxylase () catalyzes the cleavage of carbon-carbon bonds in meso 2,6 diaminoheptanedioate to produce CO2 and L-lysine, the essential amino acid. It employs the cofactor pyridoxal phosphate, also known as PLP, which participates in numerous enzymatic transamination, decarboxylation and deamination reactions. This enzyme belongs to the family of lyases, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is ''meso''-2,6-diaminoheptanedioate carboxy-lyase (L-lysine-forming).DAP-decarboxylase catalyzes the final step in the meso-diaminopimelate/lysine biosynthetic pathway. Lysine is used for protein synthesis and used in the peptidoglycan layer of Gram-positive bacteria cell walls. This enzyme is not found in humans, but the ortholog is ornithine decarboxylase. Structure DAPDC is a PLP-dependent enzyme belonging to the alanine racemase family. This enzyme is generally dimeric with each mono ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrodipicolinate Reductase
In enzymology, a 4-hydroxy-tetrahydrodipicolinate reductase () is an enzyme that catalyzes the chemical reaction :(S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate + NAD(P)+ + H2O \rightleftharpoons (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate + NAD(P)H + H+ The 3 substrates of this enzyme are (S)-2,3,4,5-tetrahydropyridine-2,6-dicarboxylate, NAD+ or NADP+, and H2O, whereas its 3 products are (2S,4S)-4-hydroxy-2,3,4,5-tetrahydrodipicolinate, NADH or NADPH, and H+. This enzyme participates in lysine biosynthesis. Nomenclature This enzyme belongs to the family of oxidoreductases, specifically those acting on CH or CH2 groups with NAD+ or NADP+ as acceptor. The systematic name A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature. A semisystematic name or semitrivial ... of this enzyme class is (S)-2,3,4,5-tetra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diaminopimelate
Diaminopimelic acid (DAP) is an amino acid, representing an epsilon-carboxy derivative of lysine. DAP is a characteristic of certain cell walls of some bacteria. DAP is often found in the peptide linkages of NAM-NAG chains that make up the cell wall of Gram-negative bacteria, gram-negative bacteria. When provided, they exhibit normal growth. When in deficiency, they still grow but with the inability to make new cell wall peptidoglycan. This is also the attachment point for Braun's lipoprotein. See also * Aspartate-semialdehyde dehydrogenase, an enzyme involved in DAP synthesis * Peptidoglycan * Pimelic acid Images References Amino acids Dicarboxylic acids Non-proteinogenic amino acids {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. It contains an α-amino group (which is in the protonated form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain lysyl ((CH2)4NH2), classifying it as a basic, charged (at physiological pH), aliphatic amino acid. It is encoded by the codons AAA and AAG. Like almost all other amino acids, the α-carbon is chiral and lysine may refer to either enantiomer or a racemic mixture of both. For the purpose of this article, lysine will refer to the biologically active enantiomer L-lysine, where the α-carbon is in the ''S'' configuration. The human body cannot synthesize lysine. It is essential in humans and must therefore be obtained from the diet. In organisms that synthesise lysine, two main biosynthetic pathways exist, the diaminopimelate and α-aminoadipate pathways, which employ distinct e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrodipicolinate Synthase
4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), ''dapA (gene)'') is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4''S'')-4-hydroxy-2,3,4,5-tetrahydro-(2''S'')-dipicolinate-forming). This enzyme catalyses the following chemical reaction : pyruvate + L-aspartate-4-semialdehyde \rightleftharpoons (2''S'',4''S'')-4-hydroxy-2,3,4,5-tetrahydrodipicolinate + H2O The reaction proceeds in three consecutive steps. Function This enzyme belongs to the family of lyases, specifically the amine-lyases, which cleave carbon-nitrogen bonds. 4-hydroxy-tetrahydrodipicolinate synthase is the key enzyme in lysine biosynthesis via the diaminopimelate pathway of prokaryotes, some phycomycetes, and higher plants. The enzyme catalyses the condensation of L-aspartate-b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |