|
TXNDC3
Thioredoxin domain-containing protein 3 (TXNDC3), also known as spermatid-specific thioredoxin-2 (Sptrx-2), is a protein that in humans is encoded by the NME8 gene (also known as the ''TXNDC3'' gene) on chromosome 7. Function This gene encodes a protein with an N-terminal thioredoxin domain and three C-terminal nucleoside diphosphate kinase ( NDK) domains, but the NDK domains are thought to be catalytically inactive. The sea urchin ortholog of this gene encodes a component of sperm outer dynein Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements importa ... arms, and the protein is implicated in ciliary function. Clinical significance Mutations in the ''TXNDC3'' gene are associated with primary ciliary dyskinesia. References Further reading * * * * * * * * External l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioredoxin Domain
Thioredoxins are small disulfide-containing redox proteins that have been found in all the kingdoms of living organisms. Thioredoxin serves as a general protein disulfide oxidoreductase. It interacts with a broad range of proteins by a redox mechanism based on reversible oxidation of 2 cysteine thiol groups to a disulfide, accompanied by the transfer of 2 electrons and 2 protons. The net result is the covalent interconversion of a disulfide and a dithiol. TR-S2 + NADPH + H+ -> TR-(SH)2 + NADP+ (1) trx-S2 + TR-(SH)2 -> trx-(SH)2 + TR-S2 (2) Protein-S2 + trx-(SH)2 -> Protein-(SH)2 + trx-S2 (3) In the NADPH-dependent protein disulfide reduction, thioredoxin reductase (TR) catalyses reduction of oxidised thioredoxin (trx) by NADPH using FAD and its redox-active disulfide (steps 1 and 2). Reduced thioredoxin then directly reduces the disulfide in the substrate protein (step 3). Protein disulfide isomerase (PDI), a resident foldase of the endoplasmic reticulum, is a multi-functional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Primary Ciliary Dyskinesia
Primary ciliary dyskinesia (PCD) is a rare, autosomal recessive genetic ciliopathy, that causes defects in the action of cilia lining the upper and lower respiratory tract, sinuses, Eustachian tube, middle ear, Fallopian tube, and flagella of sperm cells. The alternative name of "immotile ciliary syndrome" is no longer favored as the cilia do have movement, but are merely inefficient or unsynchronized. When accompanied by situs inversus the condition is known as Kartagener syndrome. Respiratory epithelial motile cilia, which resemble microscopic "hairs" (although structurally and biologically unrelated to hair), are complex organelles that beat synchronously in the respiratory tract, moving mucus toward the throat. Normally, cilia beat 7 to 22 times per second, and any impairment can result in poor mucociliary clearance, with subsequent upper and lower respiratory infection. Cilia also are involved in other biological processes (such as nitric oxide production), currently th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gene
In biology, the word gene (from , ; "...Wilhelm Johannsen coined the word gene to describe the Mendelian units of heredity..." meaning ''generation'' or ''birth'' or ''gender'') can have several different meanings. The Mendelian gene is a basic unit of heredity and the molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protein-coding genes and noncoding genes. During gene expression, the DNA is first copied into RNA. The RNA can be directly functional or be the intermediate template for a protein that performs a function. The transmission of genes to an organism's offspring is the basis of the inheritance of phenotypic traits. These genes make up different DNA sequences called genotypes. Genotypes along with environmental and developmental factors determine what the phenotypes will be. Most biological traits are under the influence of polygenes (many different genes) as well as gen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chromosome 7
Chromosome 7 is one of the 23 pairs of chromosomes in humans, who normally have two copies of this chromosome. Chromosome 7 spans about 159 million base pairs (the building material of DNA) and represents between 5 and 5.5 percent of the total DNA in cells. Genes Number of genes The following are some of the gene count estimates of human chromosome 7. Because researchers use different approaches to genome annotation their predictions of the number of genes on each chromosome varies (for technical details, see gene prediction). Among various projects, the collaborative consensus coding sequence project ( CCDS) takes an extremely conservative strategy. So CCDS's gene number prediction represents a lower bound on the total number of human protein-coding genes. Gene list The following is a partial list of genes on human chromosome 7. For complete list, see the link in the infobox on the right. Diseases and disorders The following diseases are some of those related to genes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an unbound carboxyl group, the C-terminus, and an end with an unbound amine group, the N-terminus. Proteins are naturally synthesized starting from the N-terminus and ending at the C-terminus. Function C-terminal retention signals While the N-terminus of a protein often c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nucleoside Diphosphate Kinase
Nucleoside-diphosphate kinases (NDPKs, also NDP kinase, (poly)nucleotide kinases and nucleoside diphosphokinases) are enzymes that catalyze the exchange of terminal phosphate between different nucleoside diphosphates (NDP) and triphosphates (NTP) in a reversible manner to produce nucleotide triphosphates. Many NDP serve as acceptor while NTP are donors of phosphate group. The general reaction via ping-pong mechanism is as follows: XDP + YTP ←→ XTP + YDP (X and Y each represent different nitrogenous base). NDPK activities maintain an equilibrium between the concentrations of different nucleoside triphosphates such as, for example, when guanosine triphosphate (GTP) produced in the citric acid (Krebs) cycle is converted to adenosine triphosphate (ATP). Other activities include cell proliferation, differentiation and development, signal transduction, G protein-coupled receptor, endocytosis, and gene expression. Structure NDPK are homo hexameric proteins made up of monomers app ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
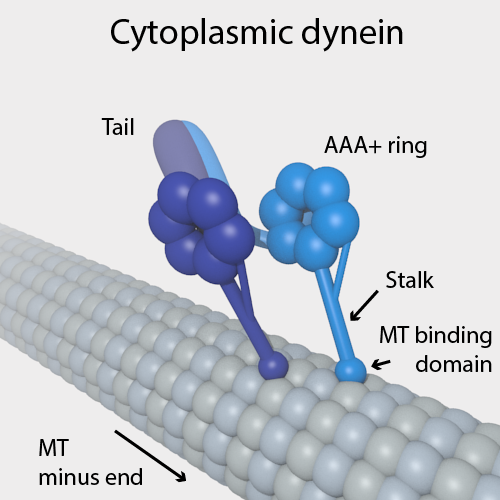
Dynein
Dyneins are a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella. All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport; thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus-end, in what is called anterograde transport. Classification Dyneins can be divided into two groups: cytoplasmic dyneins and axonemal dyneins, which are also called ciliary or flagellar dyneins. * cytoplasmic ** heavy chain: DYNC1H1, DYNC2H1 ** intermediate chain: DYNC1I1, DYNC1I2 ** light intermediate chain: DYNC1LI1, DYNC1LI2, DYNC2LI1 ** light chain: DYNLL1, DYNLL2, DYNLRB1, DYNLRB2, DYNLT1, DYNLT3 * axo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cilium
The cilium, plural cilia (), is a membrane-bound organelle found on most types of eukaryotic cell, and certain microorganisms known as ciliates. Cilia are absent in bacteria and archaea. The cilium has the shape of a slender threadlike projection that extends from the surface of the much larger cell body. Eukaryotic flagella found on sperm cells and many protozoans have a similar structure to motile cilia that enables swimming through liquids; they are longer than cilia and have a different undulating motion. There are two major classes of cilia: ''motile'' and ''non-motile'' cilia, each with a subtype, giving four types in all. A cell will typically have one primary cilium or many motile cilia. The structure of the cilium core called the axoneme determines the cilium class. Most motile cilia have a central pair of single microtubules surrounded by nine pairs of double microtubules called a 9+2 axoneme. Most non-motile cilia have a 9+0 axoneme that lacks the central pair o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arthritis Research & Therapy
''Arthritis Research & Therapy'', formerly ''Arthritis Research'', is a peer-reviewed open access medical journal covering the field of cellular and molecular mechanisms of arthritis, musculoskeletal conditions, and systemic autoimmune rheumatic diseases. It is published by BioMed Central, part of Springer Nature, and the editors-in-chief are Christopher Buckley (University of Birmingham and University of Oxford) and Harris Perlman (Northwestern University). The journal was established in 1999 as ''Arthritis Research'', obtaining its current title in. The journal's print version ceased in 2010 with volume 12, number 6, and the journal converted to an online only format. According to the ''Journal Citation Reports'', the journal has a 2017 impact factor The impact factor (IF) or journal impact factor (JIF) of an academic journal is a scientometric index calculated by Clarivate that reflects the yearly mean number of citations of articles published in the last two years in a give ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |