|
THCV Synthase
Tetrahydrocannabivarin (THCV, THV, O-4394, GWP42004) is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of a pentyl (5-carbon) group on the molecule, which makes it produce very different effects from THC. Natural occurrence THCV is prevalent in certain central Asian and southern African strains of ''Cannabis''. Chemistry Similar to THC, THCV has 7 possible double bond isomers and 30 stereoisomers (see: Tetrahydrocannabinol#Isomerism). The alternative isomer Δ8-THCV is known as a synthetic compound with a code number of O-4395, but it is not known to have been isolated from ''Cannabis'' plant material. ] Description Plants with elevated levels of propyl cannabinoids (including THCV) have been found in populations of ''Cannabis sativa'' L. ssp. ''indica'' (= ''Cannabis indica'' Lam.) from China, India, Nepal, Thailand, Afghanistan, and Pakistan, as well as southern and western Africa. THCV levels up to 53.7% of total cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration where a substance is taken through the mouth. Per os abbreviated to P.O. is sometimes used as a direction for medication to be taken orally. Many medications are taken orally because they are intended to have a systemic effect, reaching different parts of the body via the bloodstream, for example. Oral administration can be easier and less painful than other routes, such as injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients willing and able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabinoid Receptor Type 2
The cannabinoid receptor type 2, abbreviated as CB2, is a G protein-coupled receptor from the cannabinoid receptor family that in humans is encoded by the ''CNR2'' gene. It is closely related to the cannabinoid receptor type 1 (CB1), which is largely responsible for the efficacy of endocannabinoid-mediated presynaptic-inhibition, the psychoactive properties of tetrahydrocannabinol (THC), the active agent in cannabis, and other phytocannabinoids (plant cannabinoids). The principal endogenous ligand for the CB2 receptor is 2-Arachidonoylglycerol (2-AG). CB2 was cloned in 1993 by a research group from Cambridge looking for a second cannabinoid receptor that could explain the pharmacological properties of tetrahydrocannabinol. The receptor was identified among cDNAs based on its similarity in amino-acid sequence to the cannabinoid receptor type 1 (CB1) receptor, discovered in 1990. The discovery of this receptor helped provide a molecular explanation for the established effects of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases (Enzyme Commission number, EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: :RCO2H -> RH + CO2 Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Metal salts, especially copper compounds, facilitate the reaction via the intermediacy of metal carboxylate complexes. Decarboxylation of aryl carboxylates can generate the equivalent of the correspond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
THCV Synthase
Tetrahydrocannabivarin (THCV, THV, O-4394, GWP42004) is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of a pentyl (5-carbon) group on the molecule, which makes it produce very different effects from THC. Natural occurrence THCV is prevalent in certain central Asian and southern African strains of ''Cannabis''. Chemistry Similar to THC, THCV has 7 possible double bond isomers and 30 stereoisomers (see: Tetrahydrocannabinol#Isomerism). The alternative isomer Δ8-THCV is known as a synthetic compound with a code number of O-4395, but it is not known to have been isolated from ''Cannabis'' plant material. ] Description Plants with elevated levels of propyl cannabinoids (including THCV) have been found in populations of ''Cannabis sativa'' L. ssp. ''indica'' (= ''Cannabis indica'' Lam.) from China, India, Nepal, Thailand, Afghanistan, and Pakistan, as well as southern and western Africa. THCV levels up to 53.7% of total cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrocannabivarin Carboxylic Acid
Tetrahydrocannabivarin (THCV, THV, O-4394, GWP42004) is a homologue of tetrahydrocannabinol (THC) having a propyl (3-carbon) side chain instead of a pentyl (5-carbon) group on the molecule, which makes it produce very different effects from THC. Natural occurrence THCV is prevalent in certain central Asian and southern African strains of ''Cannabis''. Chemistry Similar to THC, THCV has 7 possible double bond isomers and 30 stereoisomers (see: Tetrahydrocannabinol#Isomerism). The alternative isomer Δ8-THCV is known as a synthetic compound with a code number of O-4395, but it is not known to have been isolated from ''Cannabis'' plant material. ] Description Plants with elevated levels of propyl cannabinoids (including THCV) have been found in populations of ''Cannabis sativa'' L. ssp. ''indica'' (= ''Cannabis indica'' Lam.) from China, India, Nepal, Thailand, Afghanistan, and Pakistan, as well as southern and western Africa. THCV levels up to 53.7% of total cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabigerovarin Acid
Cannabigerovarin (CBGV), the propyl homolog of cannabigerol (CBG), is a cannabinoid present in ''Cannabis''. There is no observation related to the psychoactive or psychotropic effects of CBGV when consumed or inhaled. The possible benefits of cannabigerovarin in human bodies are painkilling and anti-inflammatory properties to treat conditions like fibromyalgia and arthritis, the treatment and improvement of the dry-skin syndrome, cancer treatment by reducing the growth of cancer cells in patients who have leukemia. According to the pain-relieving effects of this natural cannabinoid, it can be helpful to treat patients who were undergoing drug exposure like chemotherapy or radiation therapy Radiation therapy or radiotherapy, often abbreviated RT, RTx, or XRT, is a therapy using ionizing radiation, generally provided as part of cancer treatment to control or kill malignant cells and normally delivered by a linear accelerator. Radi .... In addition, cannabigerol metabolis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
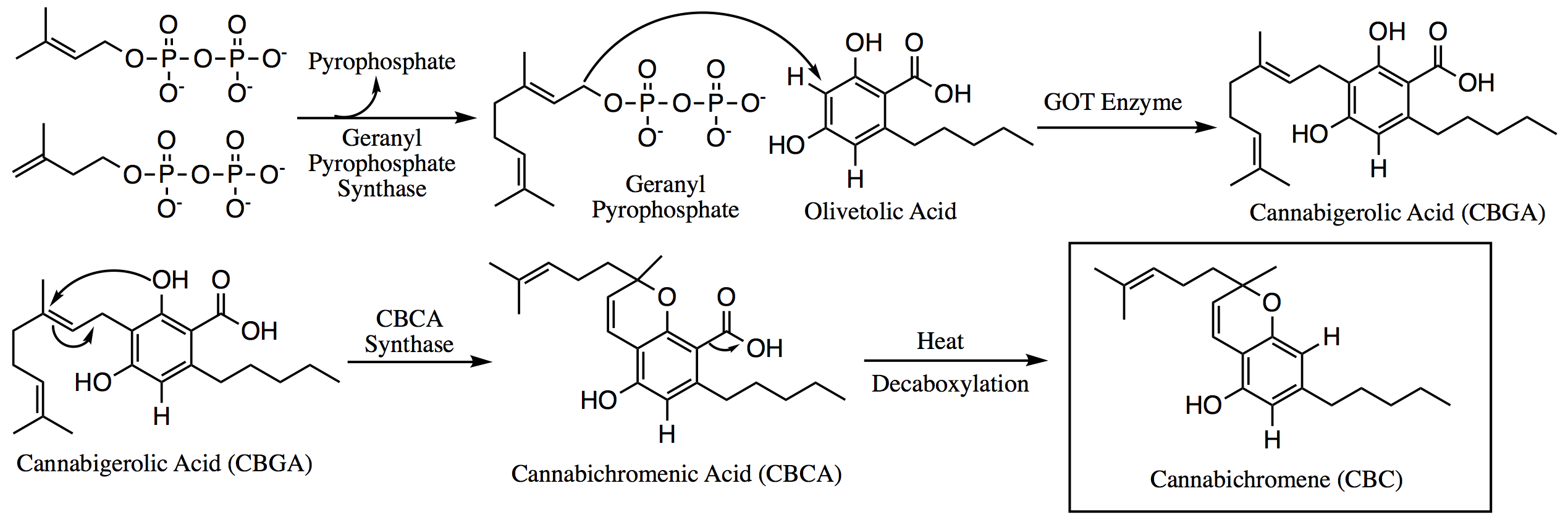
Geranyl Pyrophosphate
Geranyl pyrophosphate (GPP), also known as geranyl diphosphate (GDP), is the pyrophosphate ester of the terpenoid geraniol. Its salts are colorless. It is a precursor to many natural products. Occurrence GPP is an intermediate in the isoprenoid biosynthesis pathway that produces longer prenyl chains such as farnesyl pyrophosphate and geranylgeranyl pyrophosphate as well as many terpenes. It can be prepared in the laboratory from geraniol. Related compounds * Geraniol * Farnesyl pyrophosphate * Geranylgeranyl pyrophosphate See also * Dimethylallyltranstransferase Dimethylallyltranstransferase (DMATT), also known as farnesylpyrophosphate synthase (FPPS) or as farnesyldiphosphate synthase (FDPS), is an enzyme that in humans is encoded by the FDPS gene and catalyzes the transformation of dimethylallylpyr ... References Further reading *Kulkarni RS, Pandit SS, Chidley HG, Nagel R, Schmidt A, Gershenzon J, Pujari KH, Giri AP and Gupta VS, 2013Characterization of three n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Olivetolic Acid
Olivetolic acid is an organic compound that is an intermediate in the biosynthetic pathway of the cannabinoids in ''Cannabis sativa ''Cannabis sativa'' is an annual herbaceous flowering plant indigenous to Eastern Asia, but now of cosmopolitan distribution due to widespread cultivation. It has been cultivated throughout recorded history, used as a source of industrial fibe ...''. The ester dimer of olivetolic acid, anziaic acid, is found in lichen. References {{organic-compound-stub Benzoic acids Cannabinoids ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabigerolic Acid
Cannabigerolic acid (CBGA) is the acidic form of cannabigerol (CBG). It is a dihydroxybenzoic acid and olivetolic acid in which the hydrogen at position 3 is substituted by a geranyl group. It is a biosynthetic precursor to Delta-9-tetrahydrocannabinol, which is the principal psychoactive constituent of the ''Cannabis'' plant. It is also a diterpenoid, a polyketide, a member of resorcinols and a phytocannabinoid. It derives from an olivetolic acid. It is a conjugate acid of a cannabigerolate. In the ''Cannabis'' plant, olivetolic acid and geranyl diphosphate are synthesized into CBGA. CBGA is converted in the plant by CBCA synthase, CBDA synthase and THCA synthase into CBCA, CBDA and THCA respectively. Afterwards, THCA and CBDA can be decarboxylated into THC and CBD by drying and heating plant material. CBGA has emerging pharmacological properties; for example, it had anticonvulsant effects in a mouse model of Dravet syndrome, a form of epilepsy. Pharmacology In an ana ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabichromene
Cannabichromene (CBC), also called cannabichrome, cannanbichromene, pentylcannabichromene or cannabinochromene, is an anti-inflammatory which may contribute to the pain-killing effect of cannabis. It is one of the hundreds of cannabinoids found in the ''Cannabis'' plant, and is therefore a phytocannabinoid. It bears structural similarity to the other natural cannabinoids, including tetrahydrocannabinol (THC), tetrahydrocannabivarin (THCV), cannabidiol (CBD), and cannabinol (CBN), among others. CBC and its derivatives are as abundant as cannabinols in cannabis. It is not scheduled by the Convention on Psychotropic Substances. It is more common in tropical cannabis varieties. Biosynthesis Within the ''Cannabis'' plant, CBC occurs mainly as cannabichromenic acid (CBCA, 2-COOH-CBC, CBC-COOH). Geranyl pyrophosphate and olivetolic acid combine to produce cannabigerolic acid (CBGA; the sole intermediate for all other phytocannabinoids), which is cyclized by the enzyme CBCA synthas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cannabidiol
Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. , clinical research on CBD included studies related to anxiety, cognition, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions. Nevertheless, CBD is an herbal dietary supplement promoted with unproven claims of particular therapeutic effects. The global market size for CBD was predicted to exceed billion by 2028. Cannabidiol can be taken internally in multiple ways, including by inhaling cannabis smoke or vapor, oral, and as an aerosol spray into the cheek. It may be supplied as CBD oil containing only CBD as the active ingredient (excluding tetrahydrocannabinol HCor terpenes), CBD-dominant hemp extract oil, capsules, dried cannabis, or prescription liquid solution. CBD does not have the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |