|
T1 Holin Family
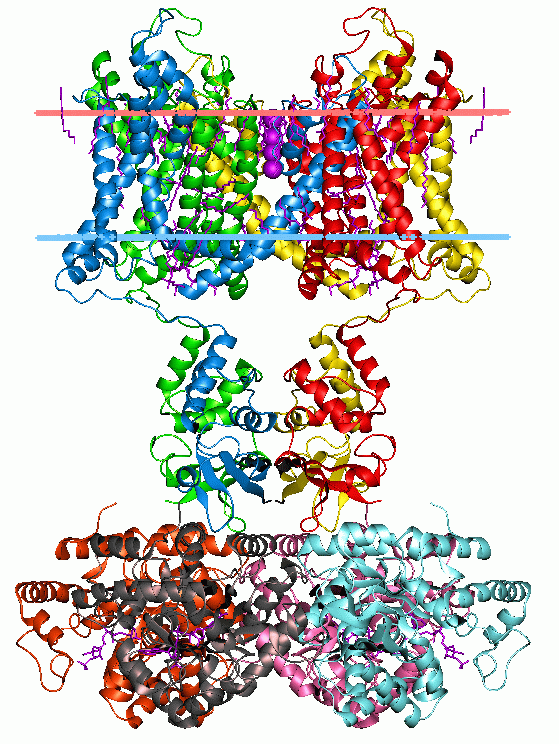
The Phage T1 Holin (T1 Holin) FamilyTC# 1.E.37 is represented in Enterobacteriales, enterobacterial phages T1, RTP and F20, ''Klebsiella'' phage KP36, and ''Escherichia'' phage ADB-2. All of these possess a putative holin that share a high level of identity. Additionally, Gp9 of ''Escherichia coli, E. coli'' phage phiE49 is similar in sequence. These proteins are short, 55 to 71 amino acyl residues (aas) in length, and exhibit a single transmembrane segment (TMS). A representative list of proteins belonging to the T1 Holin family can be found in thTransporter Classification Database See also * Holin * Lysin * Transporter Classification Database Further reading * . * . * . * . References {{CCBYSASource, sourcepath=http://tcdb.org/search/result.php?tc=1.E.37, sourcearticle=The Phage T1 Holin (T1 Holin) Family, revision=699838558 Protein families Membrane proteins Transmembrane proteins Transmembrane transporters Transport proteins Integral membrane proteins Holins ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enterobacteriales
Enterobacterales is an order of Gram-negative bacteria, Gram-negative, non-spore forming, Facultative anaerobic organism, facultatively anaerobic, rod-shaped bacteria with the class Gammaproteobacteria. The type genus of this order is ''Enterobacter.'' The name Enterobacterales is derived from the Latin term ''Enterobacter'', referring the type genus of the order and the suffix "-ales", an ending used to denote an order. Together, Enterobacterales refers to an order whose nomenclatural type is the genus ''Enterobacter''. Historical Identification and Systematics Enterobacterales was proposed in 2005 under the name "Enterobacteriales". However, the name "Enterobacteriales" was not validated according to the rules of the ''International Code of Nomenclature of Prokaryotes,'' thus it lacked standing in nomenclature, so the name was written in parentheses. "Enterobacteriales" was a monotypic order, containing only the family ''Enterobacteriaceae'', and shared its type genus ''Esch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Klebsiella
''Klebsiella'' is a genus of Gram-negative, oxidase-negative, rod-shaped bacteria with a prominent polysaccharide-based capsule. ''Klebsiella'' species are found everywhere in nature. This is thought to be due to distinct sublineages developing specific niche adaptations, with associated biochemical adaptations which make them better suited to a particular environment. They can be found in water, soil, plants, insects and other animals including humans. ''Klebsiella'' is named after German-Swiss microbiologist Edwin Klebs (1834–1913). Carl Friedlander described ''Klebsiella'' bacillus which is why it was termed Friedlander bacillus for many years. The members of the genus ''Klebsiella'' are a part of the human and animal's normal flora in the nose, mouth and intestines. The species of ''Klebsiella'' are all gram-negative and usually non-motile. They tend to be shorter and thicker when compared to others in the family Enterobacteriaceae. The cells are rods in shape and general ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia
''Escherichia'' () is a genus of Gram-negative, non- spore-forming, facultatively anaerobic, rod-shaped bacteria from the family Enterobacteriaceae. In those species which are inhabitants of the gastrointestinal tracts of warm-blooded animals, ''Escherichia'' species provide a portion of the microbially derived vitamin K for their host. A number of the species of ''Escherichia'' are pathogenic. The genus is named after Theodor Escherich, the discoverer of ''Escherichia coli''. ''Escherichia'' are facultative aerobes, with both aerobic and anaerobic growth, and an optimum temperature of 37 °C. ''Escherichia'' are usually motile by flagella, produce gas from fermentable carbohydrates, and do not decarboxylate lysine or hydrolyze arginine. Species include '' E. albertii'', '' E. fergusonii'', '' E. hermannii'', '' E. marmotae'' and most notably, the model organism and clinically relevant '' E. coli''. '' Shimwellia blattae'' was formerly classified in this genus. Pathogenesis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Escherichia Coli
''Escherichia coli'' (),Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. also known as ''E. coli'' (), is a Gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus ''Escherichia'' that is commonly found in the lower intestine of warm-blooded organisms. Most ''E. coli'' strains are harmless, but some serotypes ( EPEC, ETEC etc.) can cause serious food poisoning in their hosts, and are occasionally responsible for food contamination incidents that prompt product recalls. Most strains do not cause disease in humans and are part of the normal microbiota of the gut; such strains are harmless or even beneficial to humans (although these strains tend to be less studied than the pathogenic ones). For example, some strains of ''E. coli'' benefit their hosts by producing vitamin K2 or by preventing the colonization of the intestine by pathogenic bacteria. These mutually beneficial relationships between ''E. col ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Holin
Holins are a diverse group of small proteins produced by dsDNA bacteriophages in order to trigger and control the degradation of the host's cell wall at the end of the lytic cycle. Holins form pores in the host's cell membrane, allowing lysins to reach and degrade peptidoglycan, a component of bacterial cell walls. Holins have been shown to regulate the timing of lysis with great precision. Over 50 unrelated gene families encode holins, making them the most diverse group of proteins with common function. Together with lysins, holins are being studied for their potential use as antibacterial agents. While canonical holins act by forming large pores, pinholins such as the S protein of lambdoid phage 21 act by forming heptameric channels that depolarize the bacterial membrane. They are associated with SAR endolysins, which remain inactive in the periplasm prior to the depolarization of the membrane. Viruses that infect eukaryotic cells may use similar channel-forming proteins called ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysin
Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins. Lysins are being used as antibacterial agents due to their high effectiveness and specificity in comparison with antibiotics, which are susceptible to bacterial resistance. Structure Double-stranded DNA phage lysins tend to lie within the 25 to 40 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Families
A protein family is a group of evolutionarily related proteins. In many cases, a protein family has a corresponding gene family, in which each gene encodes a corresponding protein with a 1:1 relationship. The term "protein family" should not be confused with family as it is used in taxonomy. Proteins in a family descend from a common ancestor and typically have similar three-dimensional structures, functions, and significant sequence similarity. The most important of these is sequence similarity (usually amino-acid sequence), since it is the strictest indicator of homology and therefore the clearest indicator of common ancestry. A fairly well developed framework exists for evaluating the significance of similarity between a group of sequences using sequence alignment methods. Proteins that do not share a common ancestor are very unlikely to show statistically significant sequence similarity, making sequence alignment a powerful tool for identifying the members of protein familie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Membrane Proteins
Membrane proteins are common proteins that are part of, or interact with, biological membranes. Membrane proteins fall into several broad categories depending on their location. Integral membrane proteins are a permanent part of a cell membrane and can either penetrate the membrane (transmembrane) or associate with one or the other side of a membrane ( integral monotopic). Peripheral membrane proteins are transiently associated with the cell membrane. Membrane proteins are common, and medically important—about a third of all human proteins are membrane proteins, and these are targets for more than half of all drugs. Nonetheless, compared to other classes of proteins, determining membrane protein structures remains a challenge in large part due to the difficulty in establishing experimental conditions that can preserve the correct conformation of the protein in isolation from its native environment. Function Membrane proteins perform a variety of functions vital to the surv ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Proteins
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Transporters
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transport Proteins
A transport protein (variously referred to as a transmembrane pump, transporter, escort protein, acid transport protein, cation transport protein, or anion transport protein) is a protein that serves the function of moving other materials within an organism. Transport proteins are vital to the growth and life of all living things. There are several different kinds of transport proteins. Carrier proteins are proteins involved in the movement of ions, small molecules, or macromolecules, such as another protein, across a biological membrane. Carrier proteins are integral membrane proteins; that is, they exist within and span the membrane across which they transport substances. The proteins may assist in the movement of substances by facilitated diffusion (i.e., passive transport) or active transport. These mechanisms of movement are known as carrier-mediated transport. Each carrier protein is designed to recognize only one substance or one group of very similar substances. Research ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |