|
Type II Collagen
Type II collagen is the basis for hyaline cartilage, including the articular cartilages at joint surfaces. It is formed by homotrimers of collagen, type II, alpha 1 chains. It makes up 50% of all protein in cartilage and 85–90% of collagen of articular cartilage. Type II collagen is organised into fibrils. This fibrillar network of collagen allows the cartilage to entrap the proteoglycan aggregate, as well as providing tensile strength to the tissue. Oral administration of native type II collagen induces oral tolerance to pathological immune responses and may be useful in arthritis. See also * Type I collagen * Collagen, type III, alpha 1 Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded ... References External links * Collagens {{gene-12-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type II, Alpha 1
Collagen, type II, alpha 1 (primary osteoarthritis, spondyloepiphyseal dysplasia, congenital), also known as COL2A1, is a human gene that provides instructions for the production of the pro-alpha1(II) chain of type II collagen. Function This gene encodes the alpha-1 chain of type II collagen, a fibrillar collagen found in cartilage and the vitreous humor of the eye. Mutations in this gene are associated with achondrogenesis, chondrodysplasia, early onset familial osteoarthritis, SED congenita, Langer-Saldino achondrogenesis, Kniest dysplasia, Stickler syndrome type I, and spondyloepimetaphyseal dysplasia Strudwick type. In addition, defects in processing chondrocalcin, a calcium binding protein that is the C-propeptide of this collagen molecule, are also associated with chondrodysplasia. There are two transcripts identified for this gene. Type II collagen, which adds structure and strength to connective tissues, is found primarily in cartilage, the jelly-like substance that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
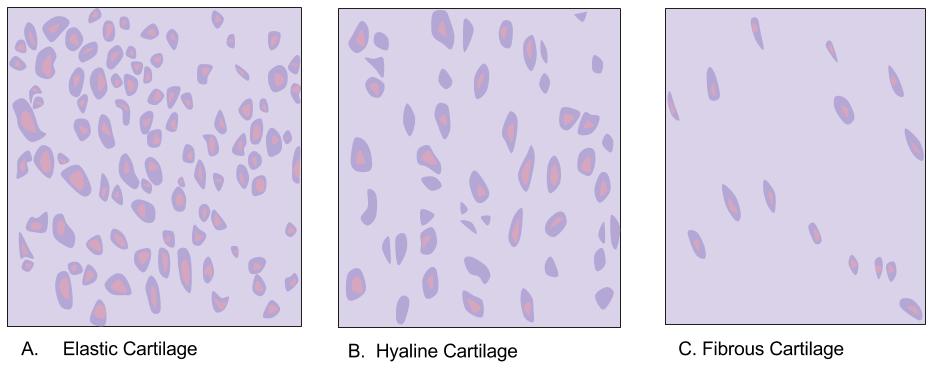
Hyaline Cartilage
Hyaline cartilage is the glass-like (hyaline) and translucent cartilage found on many joint surfaces. It is also most commonly found in the ribs, nose, larynx, and trachea. Hyaline cartilage is pearl-gray in color, with a firm consistency and has a considerable amount of collagen. It contains no nerves or blood vessels, and its structure is relatively simple. Structure Hyaline cartilage is covered externally by a fibrous membrane known as the perichondrium or, when it's along articulating surfaces, the synovial membrane. This membrane contains vessels that provide the cartilage with nutrition through diffusion. Hyaline cartilage matrix is primarily made of type II collagen and chondroitin sulphate, both of which are also found in elastic cartilage. Hyaline cartilage exists on the sternal ends of the ribs, in the larynx, trachea, and bronchi, and on the articulating surfaces of bones. It gives the structures a definite but pliable form. The presence of collagen fibres makes suc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Homotrimer
thumbnail, 400px, Trimeric form of a TNF-α mutant A homotrimer is a protein which is composed of three identical units of polypeptide. Examples * Hemagglutinin (influenza) * Spike protein (coronavirus) See also * Protein trimer In biochemistry, a protein trimer is a macromolecular complex formed by three, usually non-covalently bound, macromolecules like proteins or nucleic acids. A homotrimer would be formed by three identical molecules. A heterotrimer would be formed ... References Peptides {{protein-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type II, Alpha 1
Collagen, type II, alpha 1 (primary osteoarthritis, spondyloepiphyseal dysplasia, congenital), also known as COL2A1, is a human gene that provides instructions for the production of the pro-alpha1(II) chain of type II collagen. Function This gene encodes the alpha-1 chain of type II collagen, a fibrillar collagen found in cartilage and the vitreous humor of the eye. Mutations in this gene are associated with achondrogenesis, chondrodysplasia, early onset familial osteoarthritis, SED congenita, Langer-Saldino achondrogenesis, Kniest dysplasia, Stickler syndrome type I, and spondyloepimetaphyseal dysplasia Strudwick type. In addition, defects in processing chondrocalcin, a calcium binding protein that is the C-propeptide of this collagen molecule, are also associated with chondrodysplasia. There are two transcripts identified for this gene. Type II collagen, which adds structure and strength to connective tissues, is found primarily in cartilage, the jelly-like substance that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cartilage
Cartilage is a resilient and smooth type of connective tissue. In tetrapods, it covers and protects the ends of long bones at the joints as articular cartilage, and is a structural component of many body parts including the rib cage, the neck and the bronchial tubes, and the intervertebral discs. In other taxa, such as chondrichthyans, but also in cyclostomes, it may constitute a much greater proportion of the skeleton. It is not as hard and rigid as bone, but it is much stiffer and much less flexible than muscle. The matrix of cartilage is made up of glycosaminoglycans, proteoglycans, collagen fibers and, sometimes, elastin. Because of its rigidity, cartilage often serves the purpose of holding tubes open in the body. Examples include the rings of the trachea, such as the cricoid cartilage and carina. Cartilage is composed of specialized cells called chondrocytes that produce a large amount of collagenous extracellular matrix, abundant ground substance that is rich in pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fibril
Fibrils (from the Latin ''fibra'') are structural biological materials found in nearly all living organisms. Not to be confused with fibers or filaments, fibrils tend to have diameters ranging from 10-100 nanometers (whereas fibers are micro to milli-scale structures and filaments have diameters approximately 10-50 nanometers in size). Fibrils are not usually found alone but rather are parts of greater hierarchical structures commonly found in biological systems. Due to the prevalence of fibrils in biological systems, their study is of great importance in the fields of microbiology, biomechanics, and materials science. Structure and mechanics Fibrils are composed of linear biopolymers, and are characterized by rod-like structures with high length-to-diameter ratios. They often spontaneously arrange into helical structures. In biomechanics problems, fibrils can be characterized as classical beams with a roughly circular cross-sectional area on the nanometer scale. As such, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen
Collagen () is the main structural protein in the extracellular matrix found in the body's various connective tissues. As the main component of connective tissue, it is the most abundant protein in mammals, making up from 25% to 35% of the whole-body protein content. Collagen consists of amino acids bound together to form a triple helix of elongated fibril known as a collagen helix. It is mostly found in connective tissue such as cartilage, bones, tendons, ligaments, and skin. Depending upon the degree of mineralization, collagen tissues may be rigid (bone) or compliant (tendon) or have a gradient from rigid to compliant (cartilage). Collagen is also abundant in corneas, blood vessels, the gut, intervertebral discs, and the dentin in teeth. In muscle tissue, it serves as a major component of the endomysium. Collagen constitutes one to two percent of muscle tissue and accounts for 6% of the weight of the skeletal muscle tissue. The fibroblast is the most common cell that crea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proteoglycan
Proteoglycans are proteins that are heavily glycosylated. The basic proteoglycan unit consists of a "core protein" with one or more covalently attached glycosaminoglycan (GAG) chain(s). The point of attachment is a serine (Ser) residue to which the glycosaminoglycan is joined through a tetrasaccharide bridge (e.g. chondroitin sulfate- GlcA- Gal-Gal- Xyl-PROTEIN). The Ser residue is generally in the sequence -Ser-Gly-X-Gly- (where X can be any amino acid residue but proline), although not every protein with this sequence has an attached glycosaminoglycan. The chains are long, linear carbohydrate polymers that are negatively charged under physiological conditions due to the occurrence of sulfate and uronic acid groups. Proteoglycans occur in connective tissue. Types Proteoglycans are categorized by their relative size (large and small) and the nature of their glycosaminoglycan chains. Types include: Certain members are considered members of the "small leucine-rich proteoglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Type I Collagen
Type I collagen is the most abundant collagen of the human body. It forms large, eosinophilic fibers known as collagen fibers. It is present in scar tissue, the end product when tissue heals by repair, as well as tendons, ligaments, the endomysium of myofibrils, the organic part of bone, the dermis, the dentin, and organ capsules. Formation The gene produces the pro-alpha1(I) chain. This chain combines with another pro-alpha1(I) chain and also with a pro-alpha2(I) chain (produced by the gene) to make a molecule of type I pro-collagen. These triple-stranded, rope-like pro-collagen molecules must be processed by enzymes outside the cell. Once these molecules are processed, they arrange themselves into long, thin fibrils that cross-link to one another in the spaces around cells. The cross-links result in the formation of very strong mature type I collagen fiber. Clinical significance See Collagen, type I, alpha 1#Clinical significance Markers used to measure bone loss are not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collagen, Type III, Alpha 1
Type III Collagen is a homotrimer, or a protein composed of three identical peptide chains (monomers), each called an alpha 1 chain of type III collagen. Formally, the monomers are called collagen type III, alpha-1 chain and in humans are encoded by the gene. Type III collagen is one of the fibrillar collagens whose proteins have a long, inflexible, triple-helical domain. Protein structure and function Type III collagen is synthesized by cells as a pre-procollagen. The signal peptide is cleaved off producing a procollagen molecule. Three identical type III procollagen chains come together at the carboxy-terminal ends, and the structure is stabilized by the formation of disulphide bonds. Each individual chain folds into left-handed helix and the three chains are then wrapped together into a right-handed superhelix, the triple helix. Prior to assembling the super-helix, each monomer is subjected to a number of post-translational modifications that occur while the monomer is b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |