|
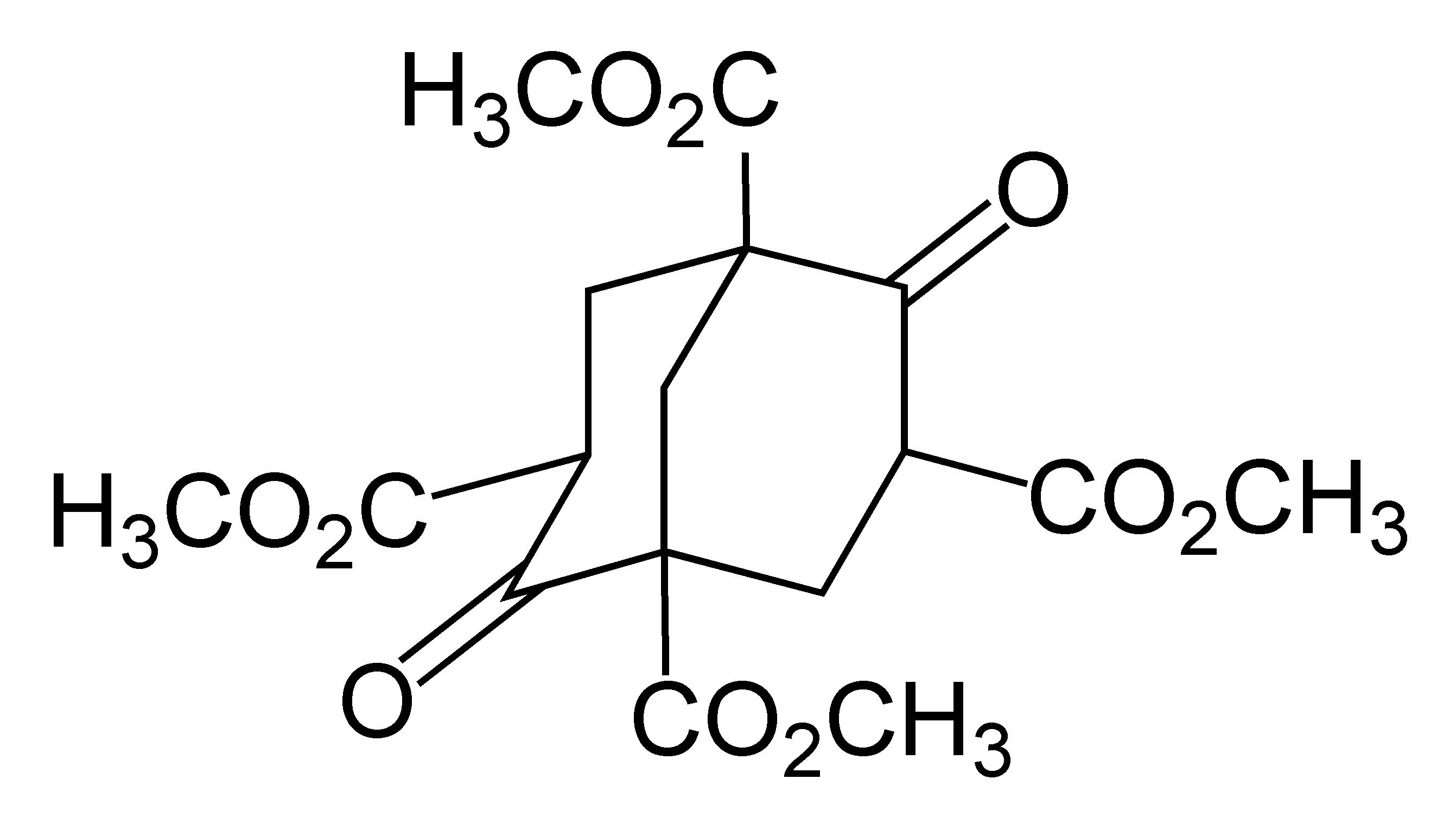
Twistane
Twistane (IUPAC name: tricyclo .4.0.03,8ecane) is an organic compound with the formula C10 H16. It is a cycloalkane and an isomer of the simplest diamondoid, adamantane, and like adamantane, is not very volatile. Twistane was named for the way its rings are permanently forced into the cyclohexane conformation known as the "twist-boat". The compound was first reported by Whitlock in 1962. Synthesis Twistane has been synthesized in a variety of ways. The original 1962 method was based on a bicyclo .2.2ctane framework. A 1967 publication concerned an intramolecular aldol condensation of a ''cis''-decalin di ketone. It is formed when basketane is hydrogenated. Symmetry The only symmetry operation in twistane is ''rotation'', and there exist three 2-fold axes as shown in the left picture. Thus the point group of twistane is D2. Although twistane has four stereocenters, it only exists as two enantiomers. This is because it is symmetric along its C2 axis. Polytwistane Polytwis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adamantane
Adamantane is an organic compound with a formula C10H16 or, more descriptively, (CH)4(CH2)6. Adamantane molecules can be described as the fusion of three cyclohexane rings. The molecule is both rigid and virtually stress-free. Adamantane is the most stable isomer of C10H16. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This similarity led to the name ''adamantane'', which is derived from the Greek ''adamantinos'' (relating to steel or diamond). It is a white solid with a camphor-like odor. It is the simplest diamondoid. The discovery of adamantane in petroleum in 1933 launched a new field of chemistry dedicated to the synthesis and properties of polyhedral organic compounds. Adamantane derivatives have found practical application as drugs, polymeric materials, and thermally stable lubricants. History and synthesis In 1924, H. Decker suggested the existence of adamantane, which he called decaterpene. The first attempted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Molecular Symmetry
Molecular symmetry in chemistry describes the symmetry present in molecules and the classification of these molecules according to their symmetry. Molecular symmetry is a fundamental concept in chemistry, as it can be used to predict or explain many of a molecule's chemical properties, such as whether or not it has a dipole moment, as well as its allowed spectroscopic transitions. To do this it is necessary to use group theory. This involves classifying the states of the molecule using the irreducible representations from the character table of the symmetry group of the molecule. Symmetry is useful in the study of molecular orbitals, with applications to the Hückel method, to ligand field theory, and to the Woodward-Hoffmann rules. Many university level textbooks on physical chemistry, quantum chemistry, spectroscopy and inorganic chemistry discuss symmetry. Another framework on a larger scale is the use of crystal systems to describe crystallographic symmetry in bulk materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Basketane
Basketane is a polycyclic alkane with the chemical formula C10H12. The name is taken from its structural similarity to a basket shape. Basketane was first synthesized in 1966, independently by Masamune and Dauben and Whalen. A patent application published in 1988 used basketane, which is a hydrocarbon, as a source material in doping thin diamond layers because of the molecule's high vapor pressure, carbon ring structure, and fewer hydrogen-to-carbon bond ratio. Chemical nomenclature In the year 1989 and before the synthesis of basketane, historic chemists were intrigued by the structural make-up of molecules, specifically those in objects seen in everyday life. Using supramolecular chemistry, molecules such as cubane and basketane were named according to their corresponding shape and historically revealed certain characteristics and personal motives of chemists at that time. Naming these uniquely shaped molecules were also done considering chemical nomenclature such as adding " ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Debye
The debye (symbol: D) (; ) is a CGS unit (a non- SI metric unit) of electric dipole momentTwo equal and opposite charges separated by some distance constitute an electric dipole. This dipole possesses an electric dipole moment whose value is given as charge times length of separation, it is a vector whose direction is in the direction of the unit vector of the position vector of the positive charge w.r.t negative charge: :p = ''q''r. named in honour of the physicist Peter J. W. Debye. It is defined as statcoulomb-centimeters.The statcoulomb is also known as the franklin or electrostatic unit of charge. :1 statC = 1 Fr = 1 esu = 1 cm3/2⋅g1/2⋅s−1. Historically the debye was defined as the dipole moment resulting from two charges of opposite sign but an equal magnitude of 10−10 statcoulomb10−10 statcoulomb corresponds to approximately 0.2083 units of elementary charge. (generally called e.s.u. (electrostatic unit) in older scientific ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does not invo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dirk Trauner
Dirk Trauner (born 17 April 1967 in Linz) is an Austrian chemist. Education and training From 1986 to 1991 Trauner studied biology and biochemistry at the University of Vienna. From 1992 to 1995 he studied chemistry at the Free University of Berlin and completed his diploma under Johann Mulzer, whom he followed to Frankfurt and subsequently Vienna as an assistant. In 1997 he completed his PhD under Mulzer at the University of Vienna summa cum laude. From 1998 to 2000 he was a postdoc under Samuel J. Danishefsky at the Memorial Sloan Kettering Cancer Center in New York City. In 2000 he moved to the University of California, Berkeley, where from 2000 to 2006 he was assistant professor and from 2006 to 2010 associate professor, with additional affiliation to the Lawrence Berkeley National Laboratory from 2005 to 2008. From 2008 to 2017 he was professor for chemical biology and genetics at the Ludwig Maximilian University of Munich. He currently resides as the Janice Cutler Cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymer
A polymer (; Greek '' poly-'', "many" + ''-mer'', "part") is a substance or material consisting of very large molecules called macromolecules, composed of many repeating subunits. Due to their broad spectrum of properties, both synthetic and natural polymers play essential and ubiquitous roles in everyday life. Polymers range from familiar synthetic plastics such as polystyrene to natural biopolymers such as DNA and proteins that are fundamental to biological structure and function. Polymers, both natural and synthetic, are created via polymerization of many small molecules, known as monomers. Their consequently large molecular mass, relative to small molecule compounds, produces unique physical properties including toughness, high elasticity, viscoelasticity, and a tendency to form amorphous and semicrystalline structures rather than crystals. The term "polymer" derives from the Greek word πολύς (''polus'', meaning "many, much") and μέρος (''meros'' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereocenter
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups creates a new stereoisomer. Stereocenters are also referred to as stereogenic centers. A stereocenter is geometrically defined as a point (location) in a molecule; a stereocenter is usually but not always a specific atom, often carbon. Stereocenters can exist on chiral or achiral molecules; stereocenters can contain single bonds or double bonds. The number of hypothetical stereoisomers can be predicted by using 2''n'', with ''n'' being the number of tetrahedral stereocenters; however, exceptions such as meso compounds can reduce the prediction to below the expected 2''n''. Chirality centers are a type of stereocenter with four different substituent groups; chirality centers are a specific subset of stereocenters because they can only ha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ketone
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bond C=O). The simplest ketone is acetone (where R and R' is methyl), with the formula . Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone. Nomenclature and etymology The word ''ketone'' is derived from ''Aketon'', an old German word for ''acetone''. According to the rules of IUPAC nomenclature, ketone names are derived by changing the suffix ''-ane'' of the parent alkane to ''-anone''. Typically, the position of the carbonyl group is denoted by a number, but traditional nonsystematic names are still generally used for the most important ketones, for example acetone and benzophenone. These nonsystematic names are considere ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decalin
Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. Isomers Decalin occurs in ''cis'' and ''trans'' forms. The ''trans'' form is energetically more stable because of fewer steric interactions. ''cis''-Decalin is a chiral molecule without a chiral center; it has a two-fold rotational symmetry axis, but no reflective symmetry. However, the chirality is canceled through a chair-flipping process that turns the molecule into its mirror image. Image:Cis-trans isomerism of decahydronaphthalene.svg, Image:cis-decalin double chair.png, 2: Image:trans-decalin double chair.png, 3: File:Cisdecalin conformations.png, 4: ''trans''-Decalin The only possible way to join the two six-membered rings in the ''trans'' position means the second ring needs to start from two equatorial bonds (blue) of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclohexane Conformation
In organic chemistry, cyclohexane conformations are any of several three-dimensional shapes adopted by molecules of cyclohexane. Because many compounds feature structurally similar six-membered rings, the structure and dynamics of cyclohexane are important prototypes of a wide range of compounds. The internal angles of a regular, flat hexagon are 120°, while the preferred angle between successive bonds in a carbon chain is about 109.5°, the tetrahedral angle (the arc cosine of −). Therefore, the cyclohexane ring tends to assume non-planar (warped) conformations, which have all angles closer to 109.5° and therefore a lower strain energy than the flat hexagonal shape. Consider the carbon atoms numbered from 1 to 6 around the ring. If we hold carbon atoms 1, 2, and 3 stationary, with the correct bond lengths and the tetrahedral angle between the two bonds, and then continue by adding carbon atoms 4, 5, and 6 with the correct bond length and the tetrahedral angle, we can var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |