|
Thiuram Disulfide
Thiuram disulfides are a class of organosulfur compounds with the formula (R2NCSS)2. Many examples are known, but popular ones include R = Me and Et. They are disulfides obtained by oxidation of the dithiocarbamates. These compounds are used in sulfur vulcanization of rubber as well as pesticides and drugs. They are typically white or pale yellow solids that are soluble in organic solvents. Preparation, structure, reactions They are prepared by the oxidation of the salts of the corresponding dithiocarbamates (e.g. sodium diethyldithiocarbamate). Typical oxidants are chlorine and hydrogen peroxide: :2 R2NCSSNa + Cl2 → (R2NCSS)2 + 2 NaCl Thiuram disulfides react with Grignard reagents to give esters of dithiocarbamic acid, as in the preparation of methyl dimethyldithiocarbamate: : e2NC(S)Ssub>2 + MeMgX → Me2NC(S)SMe + Me2NCS2MgX The compounds feature planar dithiocarbamate subunits and are linked by an S−S bond of 2.00 Å. The C(S)−N bond is sh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to P Ph3 or Ph3P. It is widely used in the synthesis of organic and organometallic compounds. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether. Preparation and structure Triphenylphosphine can be prepared in the laboratory by treatment of phosphorus trichloride with phenylmagnesium bromide or phenyllithium. The industrial synthesis involves the reaction between phosphorus trichloride, chlorobenzene, and sodium: :PCl3 + 3 PhCl + 6 Na → PPh3 + 6 NaCl Triphenylphosphine crystallizes in triclinic and monoclinic modification. In both cases, the molecule adopts a pyramidal structure with propeller-like arrangement of the three phenyl groups. Principal reactions with chalcogens, halogens, and acids Oxidation Triphenylphosphine undergoes slow ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
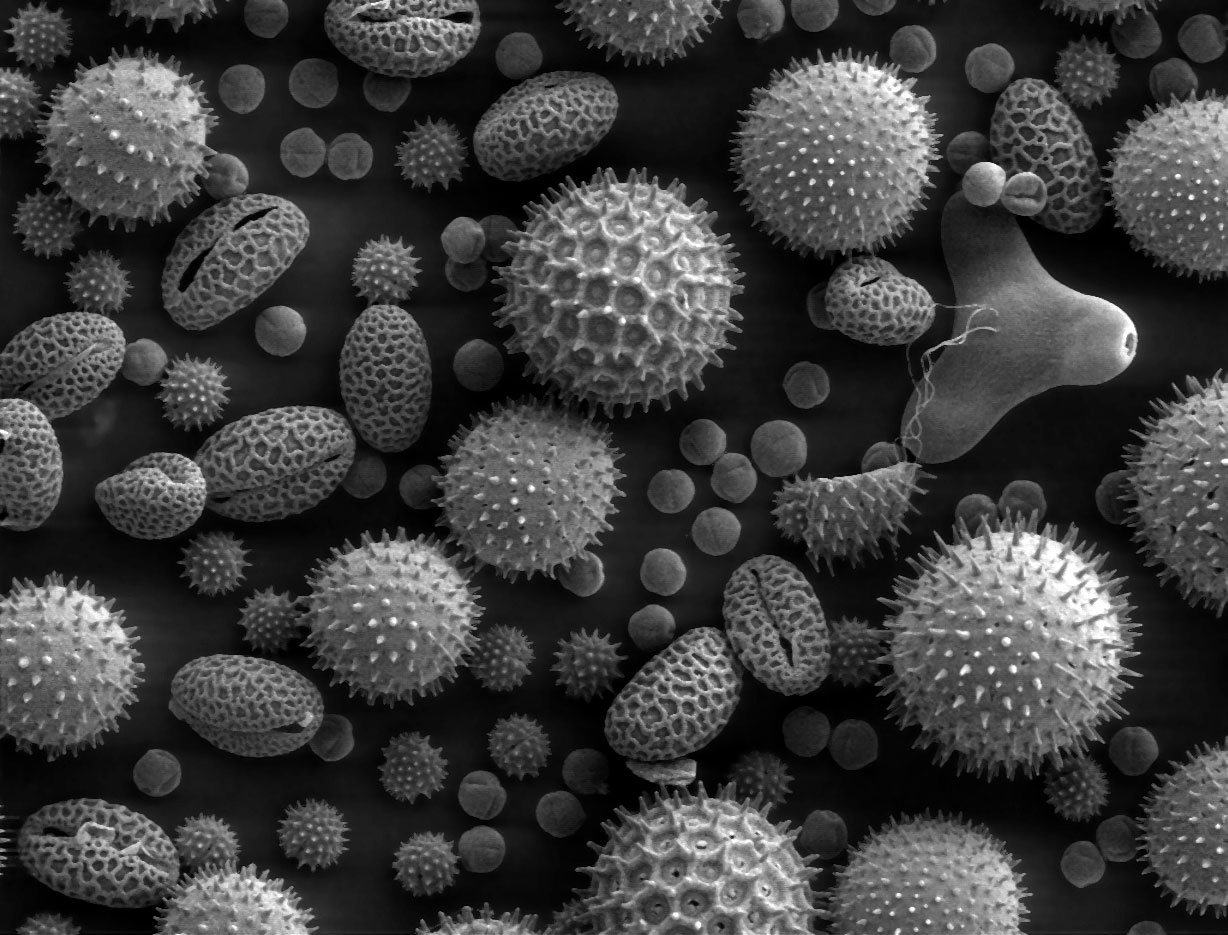
Allergen
An allergen is a type of antigen that produces an abnormally vigorous immune response in which the immune system fights off a perceived threat that would otherwise be harmless to the body. Such reactions are called allergies. In technical terms, an allergen is an antigen that is capable of stimulating a type-I hypersensitivity reaction in atopic individuals through immunoglobulin E (IgE) responses. Most humans mount significant Immunoglobulin E responses only as a defense against parasitic infections. However, some individuals may respond to many common environmental antigens. This hereditary predisposition is called atopy. In atopic individuals, non-parasitic antigens stimulate inappropriate IgE production, leading to type I hypersensitivity. Sensitivities vary widely from one person (or from one animal) to another. A very broad range of substances can be allergens to sensitive individuals. Types of allergens Allergens can be found in a variety of sources, such as dust mite e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hangover
A hangover is the experience of various unpleasant physiological and psychological effects usually following the consumption of alcohol, such as wine, beer, and liquor. Hangovers can last for several hours or for more than 24 hours. Typical symptoms of a hangover may include headache, drowsiness, concentration problems, dry mouth, dizziness, fatigue, gastrointestinal distress (e.g., vomiting, diarrhea), absence of hunger, light sensitivity, depression, sweating, nausea, hyper-excitability, irritability, and anxiety. While the causes of a hangover are still poorly understood, several factors are known to be involved including acetaldehyde accumulation, changes in the immune system and glucose metabolism, dehydration, metabolic acidosis, disturbed prostaglandin synthesis, increased cardiac output, vasodilation, sleep deprivation, and malnutrition. Beverage-specific effects of additives or by-products such as congeners in alcoholic beverages also play an important role. The symptoms ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde
Acetaldehyde (IUPAC systematic name ethanal) is an organic chemical compound with the formula CH3 CHO, sometimes abbreviated by chemists as MeCHO (Me = methyl). It is a colorless liquid or gas, boiling near room temperature. It is one of the most important aldehydes, occurring widely in nature and being produced on a large scale in industry. Acetaldehyde occurs naturally in coffee, bread, and ripe fruit, and is produced by plants. It is also produced by the partial oxidation of ethanol by the liver enzyme alcohol dehydrogenase and is a contributing cause of hangover after alcohol consumption. Pathways of exposure include air, water, land, or groundwater, as well as drink and smoke. Consumption of disulfiram inhibits acetaldehyde dehydrogenase, the enzyme responsible for the metabolism of acetaldehyde, thereby causing it to build up in the body. The International Agency for Research on Cancer (IARC) has listed acetaldehyde as a Group 1 carcinogen. Acetaldehyde is "one of the mos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetaldehyde Dehydrogenase
Acetaldehyde dehydrogenases () are dehydrogenase enzymes which catalyze the conversion of acetaldehyde into acetic acid. The oxidation of acetaldehyde to acetate can be summarized as follows: Acetaldehyde + NAD+ + Coenzyme A ↔ Acetyl-CoA + NADH + H+ In humans, there are three known genes which encode this enzymatic activity, ALDH1A1, ALDH2, and the more recently discovered ALDH1B1 (also known as ALDH5). These enzymes are members of the larger class of aldehyde dehydrogenases. The CAS number for this type of the enzyme is 028-91-5 Structure Cysteine-302 is one of three consecutive Cys residues and is crucial to the enzyme's catalytic function. The residue is alkylated by iodoacetamide in both the cytosolic and mitochondrial isozymes, with modifications to Cys-302 indicative of catalytic activity with other residues. Furthermore, the preceding sequence Gln-Gly-Gln-Cys is conserved in both isozymes for both human and horse, which is consistent with Cys-302 being crucial to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alcoholism
Alcoholism is, broadly, any drinking of alcohol (drug), alcohol that results in significant Mental health, mental or physical health problems. Because there is disagreement on the definition of the word ''alcoholism'', it is not a recognized diagnostic entity. Predominant diagnostic classifications are alcohol use disorder (DSM-5) or alcohol dependence (ICD-11); these are defined in their respective sources. Excessive alcohol use can damage all organ systems, but it particularly affects the brain, heart, liver, pancreas and immune system. Alcoholism can result in mental illness, delirium tremens, Wernicke–Korsakoff syndrome, Heart arrhythmia, irregular heartbeat, an impaired immune response, liver cirrhosis and alcohol and cancer, increased cancer risk. Drinking during pregnancy can result in fetal alcohol spectrum disorders. Women are generally more sensitive than men to the harmful effects of alcohol, primarily due to their smaller body weight, lower capacity to metaboli ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfiram
Disulfiram is a medication used to support the treatment of chronic alcoholism by producing an acute sensitivity to ethanol (drinking alcohol). Disulfiram works by inhibiting the enzyme acetaldehyde dehydrogenase, causing many of the effects of a hangover to be felt immediately following alcohol consumption. Disulfiram plus alcohol, even small amounts, produces flushing, throbbing in the head and neck, a throbbing headache, respiratory difficulty, nausea, copious vomiting, sweating, thirst, chest pain, palpitation, dyspnea, hyperventilation, fast heart rate, low blood pressure, fainting, marked uneasiness, weakness, vertigo, blurred vision, and confusion. In severe reactions there may be respiratory depression, cardiovascular collapse, abnormal heart rhythms, heart attack, acute congestive heart failure, unconsciousness, convulsions, and death. In the body, alcohol is converted to acetaldehyde, which is then broken down by acetaldehyde dehydrogenase. When the dehydrogenase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiram
Thiram is the simplest thiuram disulfide and the oxidized dimer of dimethyldithiocarbamate. It is used as a fungicide, ectoparasiticide An ectoparasiticide is an antiparasitic drug used in the treatment of ectoparasitic infestations. These drugs are used to kill the parasites that live on the body surface. Permethrin, sulfur, lindane, dicophane, benzyl benzoate, ivermectin and crot ... to prevent fungal diseases in seed and crops and similarly as an animal repellent to protect fruit trees and ornamentals from damage by rabbits, rodents and deer. It is effective against Stem gall of coriander, damping off, smut of millet, neck rot of onion, etc. Thiram has been used in the treatment of human scabies, as a sun screen and as a bactericide applied directly to the skin or incorporated into soap. Thiram is also used as a sulfur source and secondary accelerator the sulfur vulcanization of rubbers. Usage Thiram was traditionally used in apple and wine farming. Since 2010 most thiram ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiocarbamoyl Chloride
Dimethylthiocarbamoyl chloride is an organosulfur compound with the formula (CH3)2NC(S)Cl. A yellow solid, it is often encountered as a yellow syrup. It is a key reagent in the synthesis of arylthiols via the Newman-Kwart rearrangement. Synthesis and reactions Representative of other thiocarbamoyl chlorides, dimethylthiocarbamoyl chloride is electrophilic, serving as a source of R2NC(S)+.R. J. Cremlyn “An Introduction to Organosulfur Chemistry” John Wiley and Sons: Chichester (1996). It is analogous to dimethylcarbamoyl chloride (R2NC(O)Cl). Dimethylthiocarbamoyl chloride is prepared by chlorination of the related tetramethylthiuram disulfide: : e2NC(S)sub>2S2 + 3 Cl2 → 2 Me2NC(S)Cl + 2 SCl2 Dimethylthiocarbamoyl chloride reacts with dithiocarbamates (R2NCS{{su, b=2, p=−) to give thiuram sulfides 2NC(S)sub>2S. With methanethiolate, it gives methyl dimethyldithiocarbamate Methyl dimethyldithiocarbamate is the organosulfur compound with the formula (CH ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triphenylphosphine Sulfide
Triphenylphosphine sulfide (IUPAC name: triphenyl-''λ''5-phosphanethione) is the organophosphorus compound with the formula , usually written (where Ph = phenyl). It is a colourless solid, which is soluble in a variety of organic solvents. Structurally, the molecule resembles the corresponding oxide, with idealized C3 symmetry group, point group symmetry. It is weakly nucleophilic at the sulfur atom. Applications Organic synthesis Triphenylphosphine sulfide is useful for the conversion of epoxides to the corresponding episulfides: : Analytical chemistry In analytical chemistry, triphenylphosphine is used for the analysis of certain kinds of sulfur compounds. Elemental sulfur (), as occurs in some oils, and labile organosulfur compounds, such as organic trisulfides, react with triphenylphosphine to give , which can be detected by gas chromatography. References {{reflist Organophosphine sulfides Phenyl compounds ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramethylthiuram Sulfide
Tetramethylthiuram sulfide is an organosulfur compound with the formula ((CH3)2NCS)2S. It is a yellow solid that is soluble in organic solvents. It is the parent member of a large class of tetraalkylthiuram sulfides. It is used as an activator in the sulfur vulcanization of natural and butyl rubbers. Synthesis and structure It is prepared by desulfuration of tetramethylthiuram disulfides with triphenylphosphine or cyanide: :(Me2NCSS)2 + PPh3 → (Me2NCS)2S + SPPh3 According to X-ray crystallography, the molecule consists of two planar (CH3)2NCS subunits joined by a sulfide. The dihedral angle A dihedral angle is the angle between two intersecting planes or half-planes. In chemistry, it is the clockwise angle between half-planes through two sets of three atoms, having two atoms in common. In solid geometry, it is defined as the uni ... between the subunits is close to 90°. References Organosulfur compounds {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |


_-_Foto_Giovanni_Dall'Orto%2C_6-Dec-2007-cropped.jpg)

