|
Stork Enamine Reaction
The Stork enamine alkylation involves the addition of an enamine to a Michael acceptor (e.g, an α,β -unsaturated carbonyl compound) or another electrophilic alkylation reagent to give an alkylated iminium product, which is hydrolyzed by dilute aqueous acid to give the alkylated ketone or aldehyde. Since enamines are generally produced from ketones or aldehydes, this overall process (known as the Stork enamine synthesis) constitutes a selective monoalkylation of a ketone or aldehyde, a process that may be difficult to achieve directly. The Stork enamine synthesis: # formation of an enamine from a ketone # addition of the enamine to an alpha, beta-unsaturated aldehyde or ketone # hydrolysis of the enamine back to a ketone The reaction also applies to acyl halides as electrophiles, which results in the formation of 1,3-diketones (Stork acylation). It is also effective for activated sp3 alkyl electrophiles, including benzylic, allylic/propargylic, α-carbonyl (e.g., bromoacet ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enamine
An enamine is an unsaturated compound derived by the condensation of an aldehyde or ketone with a secondary amine. Enamines are versatile intermediates. : The word "enamine" is derived from the affix ''en''-, used as the suffix of alkene, and the root ''amine''. This can be compared with enol, which is a functional group containing both alkene (''en''-) and alcohol (-''ol''). Enamines are considered to be nitrogen analogs of enols. If one of the nitrogen substituents is a hydrogen atom, H, it is the tautomeric form of an imine. This usually will rearrange to the imine; however there are several exceptions (such as aniline). The enamine-imine tautomerism may be considered analogous to the keto-enol tautomerism. In both cases, a hydrogen atom switches its location between the heteroatom (oxygen or nitrogen) and the second carbon atom. Enamines are both good nucleophiles and good bases. Their behavior as carbon-based nucleophiles is explained with reference to the following reson ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyl Halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the substance and w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
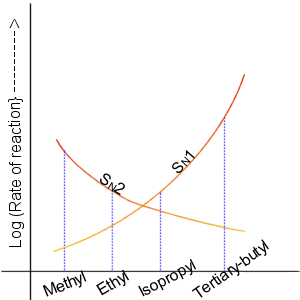
Nucleophilic Displacement
In chemistry, a nucleophilic substitution is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile). The molecule that contains the electrophile and the leaving functional group is called the substrate. The most general form of the reaction may be given as the following: :\text\mathbf + \ce + \text\mathbf The electron pair (:) from the nucleophile (Nuc) attacks the substrate () and bonds with it. Simultaneously, the leaving group (LG) departs with an electron pair. The principal product in this case is . The nucleophile may be electrically neutral or negatively charged, whereas the substrate is typically neutral or positively charged. An example of nucleophilic substitution is the hydrolysis of an alkyl bromide, R-Br under basic conditions, where the attacking nucleophile is hydroxyl () and the leaving group is bromide (). :R-Br + OH- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
A Grignard reagent or Grignard compound is a chemical compound with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon-carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. In this aspect, they are similar to organolithium reagents. Pure Grignard reagents are extremely reactive solids. They are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran; which are relatively stable as long as water is excluded. In such a medium, a Grignard reagent is invariably present as a complex with the magnesium atom conn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent such as an alkyl or aryl group (these may respectively be called alkylamines and arylamines; amines in which both types of substituent are attached to one nitrogen atom may be called alkylarylamines). Important amines include amino acids, biogenic amines, trimethylamine, and aniline; Inorganic derivatives of ammonia are also called amines, such as monochloramine (). The substituent is called an amino group. Compounds with a nitrogen atom attached to a carbonyl group, thus having the structure , are called amides and have different chemical properties from amines. Classification of amines Amines can be classified according to the nature and number of substituents on nitrogen. Aliphatic amines contain only H and alkyl substituents. A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylimino-de-oxo-bisubstitution
In organic chemistry, alkylimino-de-oxo-bisubstitution is the organic reaction of carbonyl compounds with amines to imines. The reaction name is based on the IUPAC Nomenclature for Transformations. The reaction is acid catalyzed and the reaction type is nucleophilic addition of the amine to the carbonyl compound followed by transfer of a proton from nitrogen to oxygen to a stable hemiaminal or carbinolamine. With primary amines water is lost in an elimination reaction to an imine. With aryl amines especially stable Schiff bases are formed. Reaction mechanism The reaction steps are reversible reactions and the reaction is driven to completion by removal of water e.g. by azeotropic distillation, molecular sieves or titanium tetrachloride. Primary amines react through an unstable hemiaminal intermediate which then splits off water. : Secondary amines do not lose water easily because they do not have a proton available and instead they often react further to an aminal: : or whe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bonds. Imines are common in synthetic and naturally occurring compounds and they participate in many reactions. Structure For ketimines and aldimines, respectively, the five core atoms (C2C=NX and C(H)C=NX, X = H or C) are coplanar. Planarity results from the sp2-hybridization of the mutually double-bonded carbon and the nitrogen atoms. The C=N distance is 1.29-1.31 Å for nonconjugated imines and 1.35 Å for conjugated imines. By contrast, C-N distances in amines and nitriles are 1.47 and 1.16 Å, respectively. Rotation about the C=N bond is slow. Using NMR spectroscopy, both E- and Z-isomers of aldimines have been detected. Owing to steric effects, the E isomer is favored. Nomenclature and classification The term "imine" was coine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stork C Alkylation
Storks are large, long-legged, long-necked wading birds with long, stout bills. They belong to the family called Ciconiidae, and make up the order Ciconiiformes . Ciconiiformes previously included a number of other families, such as herons and ibises, but those families have been moved to other orders. Storks dwell in many regions and tend to live in drier habitats than the closely related herons, spoonbills and ibises; they also lack the powder down that those groups use to clean off fish slime. Bill-clattering is an important mode of communication at the nest. Many species are migratory. Most storks eat frogs, fish, insects, earthworms, small birds and small mammals. There are 19 living species of storks in six genera. Various terms are used to refer to groups of storks, two frequently used ones being a ''muster'' of storks and a ''phalanx'' of storks. Storks tend to use soaring, gliding flight, which conserves energy. Soaring requires thermal air currents. Ottomar Ansch� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that carries a partial positive charge, or have an atom that does not have an octet of electrons. Electrophiles mainly interact with nucleophiles through addition and substitution reactions. Frequently seen electrophiles in organic syntheses include cations such as H+ and NO+, polarized neutral molecules such as HCl, alkyl halides, acyl halides, and carbonyl compounds, polarizable neutral molecules such as Cl2 and Br2, oxidizing agents such as organic peracids, chemical species that do not satisfy the octet rule such as carbenes and radicals, and some Lewis acids such as BH3 and DIBAL. Organic chemistry Addition of halogens These occur between alkenes and electrophiles, often halogens as in halogen addition reactions. Common reactions i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gilbert Stork
Gilbert Stork (December 31, 1921 – October 21, 2017) was an organic chemist. For a quarter of a century he was the Eugene Higgins Professor of Chemistry Emeritus at Columbia University. He is known for making significant contributions to the total synthesis of natural products, including a lifelong fascination with the synthesis of quinine. In so doing he also made a number of contributions to mechanistic understanding of reactions, and performed pioneering work on enamine chemistry, leading to development of the Stork enamine alkylation. It is believed he was responsible for the first planned stereocontrolled synthesis as well as the first natural product to be synthesised with high stereoselectivity. Stork was also an accomplished mentor of young chemists and many of his students have gone on to make significant contributions in their own right. Early years Gilbert Stork was born in the Ixelles municipality of Brussels, Belgium on December 31, 1921. The oldest of 3 childre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |




_(12011503884).jpg)
