|
Spitz (protein)
Spitz is a protein in fruit flies which is the major activator of Epidermal Growth Factor Receptor (EGFR). Function Spitz is produced as a transmembrane protein in the endoplasmic reticulum. There it associates with a cargo receptor called ''Star'' and is trafficked to the Golgi. In the Golgi, Spitz is cleaved by a protease called ''Rhomboid'', which releases Spitz to be trafficked to the cell membrane and released out of the cell. From here it can bind EGFR on the surface of other cells, activating the receptor. Alternatively, Spitz can be bound and inactivated by Argos, inhibiting EGFR activation. Spitz is responsible for activating signaling of the ''Drosophila'' epidermal growth factor receptor (DER) and is involved in the development of the embryos, eyes, and wings of fruit flies. Spitz can be sequestered and prevented from binding to DER by the protein Argos (Aos) which then inhibits the epidermal growth factor receptor pathway. Over-expression of epidermal growth factor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called peptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residue ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster
''Drosophila melanogaster'' is a species of fly (the taxonomic order Diptera) in the family Drosophilidae. The species is often referred to as the fruit fly or lesser fruit fly, or less commonly the "vinegar fly" or "pomace fly". Starting with Charles W. Woodworth's 1901 proposal of the use of this species as a model organism, ''D. melanogaster'' continues to be widely used for biological research in genetics, physiology, microbial pathogenesis, and life history evolution. As of 2017, five Nobel Prizes have been awarded to drosophilists for their work using the insect. ''D. melanogaster'' is typically used in research owing to its rapid life cycle, relatively simple genetics with only four pairs of chromosomes, and large number of offspring per generation. It was originally an African species, with all non-African lineages having a common origin. Its geographic range includes all continents, including islands. ''D. melanogaster'' is a common pest in homes, restaurants, and othe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faint Little Ball
Faint little ball (flb) is a ''Drosophila'' gene that encodes the ''Drosophila'' epidermal growth factor receptor (DER) homolog. The gene is also called ''torpedo'' and ''Ellipse''. The gene is located at 3-26 of the ''Drosophila melanogaster'' genome. It is named ''faint little ball'' because when the gene is mutated the embryo forms a ball of dorsal hypoderm. ''flb'' is necessary for several processes to occur during embryonic development, specifically in central nervous system development. It is expressed as quickly as 4 hours after fertilization of the egg. The peak of expression of the ''flb'' gene is between 4–8 hours into development. In all processes that are facilitated by ''flb'' the same signal transduction pathway is used. ''Drosophila'' EGF receptor is involved in the development of embryos as well as larvae/pupae's wings, eyes, legs and ovaries. Interactions Whether looking at development in embryos or larvae/pupae, DER relies on several ligands to carry out its f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Transmembrane Protein
A transmembrane protein (TP) is a type of integral membrane protein that spans the entirety of the cell membrane. Many transmembrane proteins function as gateways to permit the transport of specific substances across the membrane. They frequently undergo significant conformational changes to move a substance through the membrane. They are usually highly hydrophobic and aggregate and precipitate in water. They require detergents or nonpolar solvents for extraction, although some of them (beta-barrels) can be also extracted using denaturing agents. The peptide sequence that spans the membrane, or the transmembrane segment, is largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified as single-span (or bitopic) or multi-span (polytopic). Some other integral membrane proteins are called monotopic, meaning that they are also permanently attached to the membrane, but do not pass ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endoplasmic Reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum (RER), and smooth endoplasmic reticulum (SER). The endoplasmic reticulum is found in most eukaryotic cells and forms an interconnected network of flattened, membrane-enclosed sacs known as cisternae (in the RER), and tubular structures in the SER. The membranes of the ER are continuous with the outer nuclear membrane. The endoplasmic reticulum is not found in red blood cells, or spermatozoa. The two types of ER share many of the same proteins and engage in certain common activities such as the synthesis of certain lipids and cholesterol. Different types of cells contain different ratios of the two types of ER depending on the activities of the cell. RER is found mainly toward the nucleus of cell and SER towards the cell membrane or plasma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
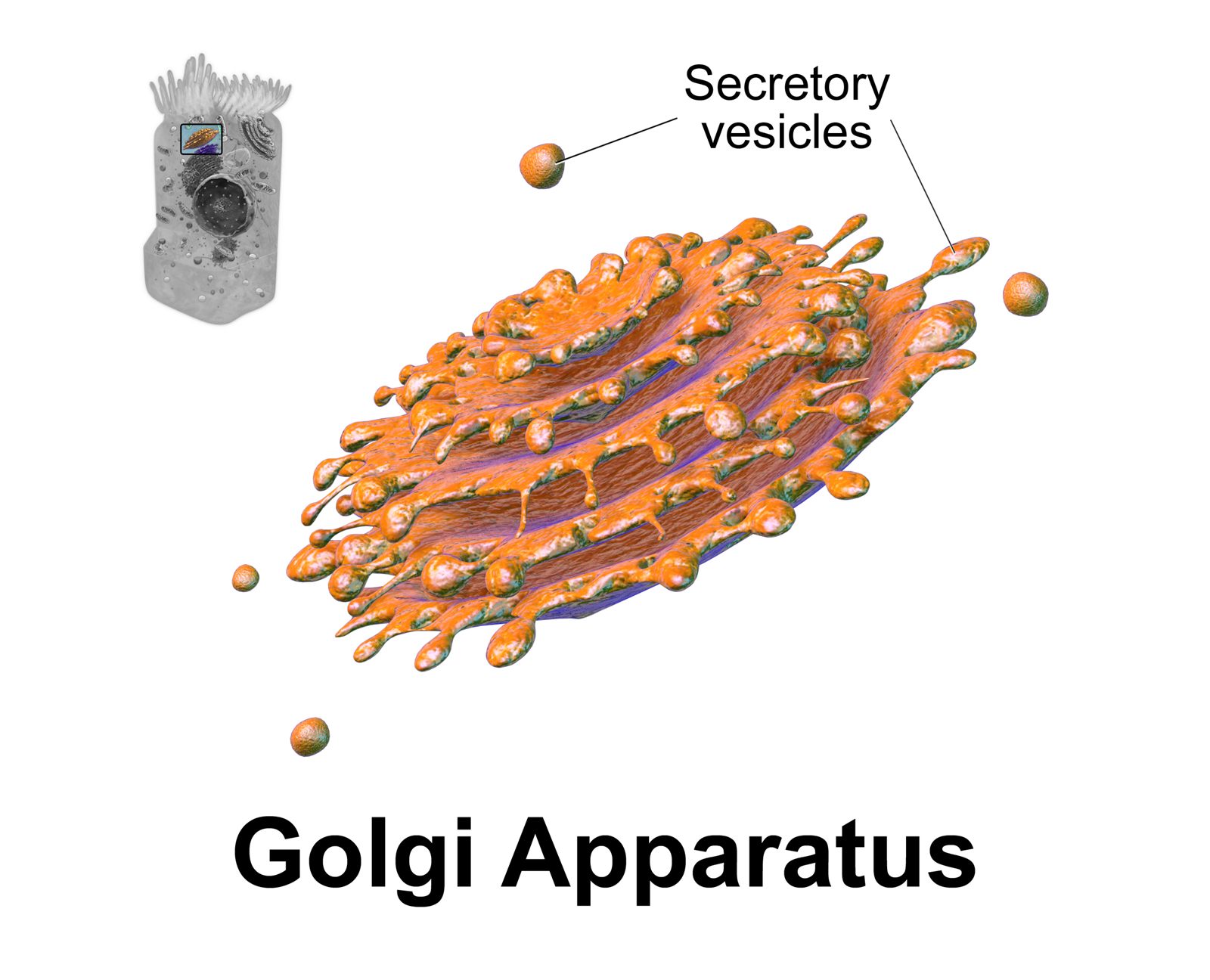
Golgi Apparatus
The Golgi apparatus (), also known as the Golgi complex, Golgi body, or simply the Golgi, is an organelle found in most eukaryotic cells. Part of the endomembrane system in the cytoplasm, it packages proteins into membrane-bound vesicles inside the cell before the vesicles are sent to their destination. It resides at the intersection of the secretory, lysosomal, and endocytic pathways. It is of particular importance in processing proteins for secretion, containing a set of glycosylation enzymes that attach various sugar monomers to proteins as the proteins move through the apparatus. It was identified in 1897 by the Italian scientist Camillo Golgi and was named after him in 1898. Discovery Owing to its large size and distinctive structure, the Golgi apparatus was one of the first organelles to be discovered and observed in detail. It was discovered in 1898 by Italian physician Camillo Golgi during an investigation of the nervous system. After first observing it under his ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protease
A protease (also called a peptidase, proteinase, or proteolytic enzyme) is an enzyme that catalyzes (increases reaction rate or "speeds up") proteolysis, breaking down proteins into smaller polypeptides or single amino acids, and spurring the formation of new protein products. They do this by cleaving the peptide bonds within proteins by hydrolysis, a reaction where water breaks bonds. Proteases are involved in many biological functions, including digestion of ingested proteins, protein catabolism (breakdown of old proteins), and cell signaling. In the absence of functional accelerants, proteolysis would be very slow, taking hundreds of years. Proteases can be found in all forms of life and viruses. They have independently evolved multiple times, and different classes of protease can perform the same reaction by completely different catalytic mechanisms. Hierarchy of proteases Based on catalytic residue Proteases can be classified into seven broad groups: * Serine protease ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Argos (EGFR Inhibitor)
Argos is a secreted protein that is an inhibitor of the epidermal growth factor receptor (EGFR) pathway in ''Drosophila melanogaster''. Argos inhibits the EGFR pathway by sequestering the EGFR ligand Spitz. Argos binds the epidermal growth factor domain of Spitz, preventing interaction between Spitz and EGFR. Argos does not directly interact with EGFR. Argos represents the first example of ligand sequestration as a mechanism of inhibition in the ErbB (EGFR) family. Function Argos is secreted from cells in ''D. melanogaster''. Outside the cell, it binds the EGFR-activator Spitz, preventing it from binding and activating EGFR. ''Drosophila'' with mutations that inactivate Argos have deformed eyes with extra photoreceptors and a small optic lobe due to disruption of EGFR's role in eye development. The name of the gene derives from the phenotype of mutant flies with eye defects and refers to Argus Panoptes. Structure Crystallographic studies have revealed that Argos does not contai ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epidermal Growth Factor Receptor (EGFR) With Spitz Protein
The epidermal growth factor receptor (EGFR; ErbB-1; HER1 in humans) is a transmembrane protein that is a receptor for members of the epidermal growth factor family (EGF family) of extracellular protein ligands. The epidermal growth factor receptor is a member of the ErbB family of receptors, a subfamily of four closely related receptor tyrosine kinases: EGFR (ErbB-1), HER2/neu (ErbB-2), Her 3 (ErbB-3) and Her 4 (ErbB-4). In many cancer types, mutations affecting EGFR expression or activity could result in cancer. Epidermal growth factor and its receptor was discovered by Stanley Cohen of Vanderbilt University. Cohen shared the 1986 Nobel Prize in Medicine with Rita Levi-Montalcini for their discovery of growth factors. Deficient signaling of the EGFR and other receptor tyrosine kinases in humans is associated with diseases such as Alzheimer's, while over-expression is associated with the development of a wide variety of tumors. Interruption of EGFR signalling, either by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residues Binding Spitz Protein And EGFR (right Side)
Residue may refer to: Chemistry and biology * An amino acid, within a peptide chain * Crop residue, materials left after agricultural processes * Pesticide residue, refers to the pesticides that may remain on or in food after they are applied to food crops * Petroleum residue, the heavier fractions of crude oil that fail to vaporize in an oil refinery * Residue (chemistry), materials remaining after a physical separation process, or by-products of a chemical reaction Mathematics * Residue (complex analysis), complex number describing the behavior of line integrals of a meromorphic function around a singularity * Some coefficient involved in partial fraction decomposition * A remainder in modular arithmetic Other * A variant title of a British folk song also known as "Levy-Dew" and "New Year Carol" * Residuum (geology), often used to refer to the soil and subsoil that forms as the result of long weathering over carbonate bedrock * Residue (law), portion of the testa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Residues Binding Spitz Protein And EGFR (left Side)
Residue may refer to: Chemistry and biology * An amino acid, within a peptide chain * Crop residue, materials left after agricultural processes * Pesticide residue, refers to the pesticides that may remain on or in food after they are applied to food crops * Petroleum residue, the heavier fractions of crude oil that fail to vaporize in an oil refinery * Residue (chemistry), materials remaining after a physical separation process, or by-products of a chemical reaction Mathematics * Residue (complex analysis), complex number describing the behavior of line integrals of a meromorphic function around a singularity * Some coefficient involved in partial fraction decomposition * A remainder in modular arithmetic Other * A variant title of a British folk song also known as "Levy-Dew" and "New Year Carol" * Residuum (geology), often used to refer to the soil and subsoil that forms as the result of long weathering over carbonate bedrock * Residue (law), portion of the testa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TGF Alpha
Transforming growth factor alpha (TGF-α) is a protein that in humans is encoded by the TGFA gene. As a member of the epidermal growth factor (EGF) family, TGF-α is a mitogenic polypeptide. The protein becomes activated when binding to receptors capable of protein kinase activity for cellular signaling. TGF-α is a transforming growth factor that is a ligand for the epidermal growth factor receptor, which activates a signaling pathway for cell proliferation, differentiation and development. This protein may act as either a transmembrane-bound ligand or a soluble ligand. This gene has been associated with many types of cancers, and it may also be involved in some cases of cleft lip/palate. Synthesis TGF-α is synthesized internally as part of a 160 (human) or 159 (rat) amino acid transmembrane precursor.Ferrer, I.; Alcantara, S.; Ballabriga, J.; Olive, M.; Blanco, R.; Rivera, R.; Carmona, M.; Berruezo, M.; Pitarch, S.; Planas, A. Transforming growth factor- α (TGF-α) and epide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
_with_Spitz_protein.png)