|
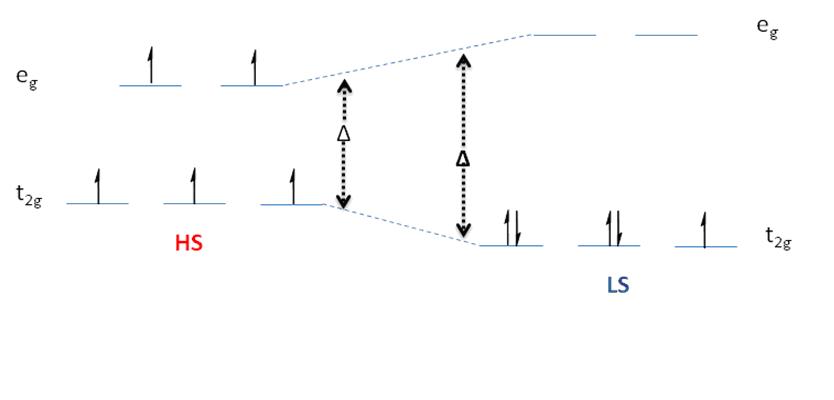
Spin Crossover
Spin crossover (SCO) is a phenomenon that occurs in some metal complexes wherein the spin state of the complex changes due to an external stimulus. The stimuli can include temperature or pressure. Spin crossover is sometimes referred to as spin transition or spin equilibrium behavior. The change in spin state usually involves interchange of low spin (LS) and high spin (HS) configuration. Spin crossover is commonly observed with first row transition metal complexes with a d4 through d7 electron configuration in an octahedral ligand geometry. Spin transition curves typically plot the high-spin molar fraction against temperature. Often a gradual spin transition is followed by an abrupt (ΔT = 10K) transition with hysteresis and a two-step transition. The abruptness with hysteresis indicates cooperativity, or “communication”, between neighboring metal complexes. In the latter case, the material is bistable and can exist in the two different spin states with a different range of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coordination Complex
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many metal-containing compounds, especially those that include transition metals (elements like titanium that belong to the Periodic Table's d-block), are coordination complexes. Nomenclature and terminology Coordination complexes are so pervasive that their structures and reactions are described in many ways, sometimes confusingly. The atom within a ligand that is bonded to the central metal atom or ion is called the donor atom. In a typical complex, a metal ion is bonded to several donor atoms, which can be the same or different. A polydentate (multiple bonded) ligand is a molecule or ion that bonds to the central atom through several of the ligand's atoms; ligands with 2, 3, 4 or even 6 bonds to the central atom are common. These compl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Spin States (d Electrons)
Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity only applies to first row metals, because second- and third-row metals are invariably low-spin. These configurations can be understood through the two major models used to describe coordination complexes; crystal field theory and ligand field theory (a more advanced version based on molecular orbital theory). High-spin vs. low-spin Octahedral complexes The Δ splitting of the ''d'' orbitals plays an important role in the electron spin state of a coordination complex. Three factors affect Δ: the period (row in periodic table) of the metal ion, the charge of the metal ion, and the field strength of the complex's ligands as described by the spectrochemical series. Only octahedral complexes of first row transition metals adopt high ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hysteresis
Hysteresis is the dependence of the state of a system on its history. For example, a magnet may have more than one possible magnetic moment in a given magnetic field, depending on how the field changed in the past. Plots of a single component of the moment often form a loop or hysteresis curve, where there are different values of one variable depending on the direction of change of another variable. This history dependence is the basis of memory in a hard disk drive and the remanence that retains a record of the Earth's magnetic field magnitude in the past. Hysteresis occurs in ferromagnetic and ferroelectric materials, as well as in the deformation of rubber bands and shape-memory alloys and many other natural phenomena. In natural systems it is often associated with irreversible thermodynamic change such as phase transitions and with internal friction; and dissipation is a common side effect. Hysteresis can be found in physics, chemistry, engineering, biology, and economics. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
LIESST
In chemistry and physics, LIESST (Light-Induced Excited Spin-State Trapping) is a method of changing the electronic spin state of a compound by means of irradiation with light.Phillip Gūtlich, Light Induced Electronic Spin State Trapping (LIESST) Many complexes with d4-d7 are capable of |
Iron Tris(dimethyldithiocarbamate)
Iron tris(dimethyldithiocarbamate) is the coordination complex of iron with dimethyldithiocarbamate with the formula Fe(S2CNMe2)3 (Me = methyl). It is marketed as a fungicide. Synthesis, structure, bonding Iron tris(dithiocarbamate)s are typically are prepared by salt metathesis reactions. Iron tris(dimethyldithiocarbamate) is an octahedral coordination complex of iron(III) with D3 symmetry. Spin crossover (SCO) was first observed in 1931 by Cambi ''et al.'' who discovered anomalous magnetic behavior for the tris(N,N-dialkyldithiocarbamatoiron(III) complexes. The spin states of these complexes are sensitive to the nature of the amine substituents. Iron tris(dithiocarbamate)s characteristically react with nitric oxide to give Fe(dtc)2NO. This efficient chemical trapping reaction provides a means to detect NO. Reflecting the strongly donating properties of dithiocarbamate ligands, iron tris(dithiocarbamate)s oxidize at relatively mild potentials to give isolable iron(IV) deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Susceptibility
In electromagnetism, the magnetic susceptibility (Latin: , "receptive"; denoted ) is a measure of how much a material will become magnetized in an applied magnetic field. It is the ratio of magnetization (magnetic moment per unit volume) to the applied magnetizing field intensity . This allows a simple classification, into two categories, of most materials' responses to an applied magnetic field: an alignment with the magnetic field, , called paramagnetism, or an alignment against the field, , called diamagnetism. Magnetic susceptibility indicates whether a material is attracted into or repelled out of a magnetic field. Paramagnetic materials align with the applied field and are attracted to regions of greater magnetic field. Diamagnetic materials are anti-aligned and are pushed away, toward regions of lower magnetic fields. On top of the applied field, the magnetization of the material adds its own magnetic field, causing the field lines to concentrate in paramagnetism, or be excl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mössbauer Spectroscopy
Mössbauer spectroscopy is a spectroscopic technique based on the Mössbauer effect. This effect, discovered by Rudolf Mössbauer (sometimes written "Moessbauer", German: "Mößbauer") in 1958, consists of the nearly recoil-free emission and absorption of nuclear gamma rays in solids. The consequent nuclear spectroscopy method is exquisitely sensitive to small changes in the chemical environment of certain nuclei. Typically, three types of nuclear interactions may be observed: the isomer shift due to differences in nearby electron densities (also called the chemical shift in older literature), quadrupole splitting due to atomic-scale electric field gradients; and magnetic Zeeman splitting due to non-nuclear magnetic fields. Due to the high energy and extremely narrow line widths of nuclear gamma rays, Mössbauer spectroscopy is a highly sensitive technique in terms of energy (and hence frequency) resolution, capable of detecting changes of just a few parts in 1011. It is a me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
FT-IR
Fourier-transform infrared spectroscopy (FTIR) is a technique used to obtain an infrared Electromagnetic spectrum, spectrum of Absorption (electromagnetic radiation), absorption or Emission (electromagnetic radiation), emission of a solid, liquid, or gas. An FTIR spectrometer simultaneously collects high-resolution spectral data over a wide spectral range. This confers a significant advantage over a Dispersion (optics), dispersive spectrometer, which measures intensity over a narrow range of wavelengths at a time. The term ''Fourier-transform infrared spectroscopy'' originates from the fact that a Fourier transform (a mathematical process) is required to convert the raw data into the actual spectrum. Conceptual introduction The goal of absorption spectroscopy techniques (FTIR, Ultraviolet-visible spectroscopy, ultraviolet-visible ("UV-vis") spectroscopy, etc.) is to measure how much light a sample absorbs at each wavelength. The most straightforward way to do this, the "dispe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Raman Spectroscopy
Raman spectroscopy () (named after Indian physicist C. V. Raman) is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified. Raman spectroscopy relies upon inelastic scattering of photons, known as Raman scattering. A source of monochromatic light, usually from a laser in the visible, near infrared, or near ultraviolet range is used, although X-rays can also be used. The laser light interacts with molecular vibrations, phonons or other excitations in the system, resulting in the energy of the laser photons being shifted up or down. The shift in energy gives information about the vibrational modes in the system. Infrared spectroscopy typically yields similar yet complementary information. Typically, a sample is illuminated with a laser beam. Electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |