|
Solid Nitrogen
Solid nitrogen is a number of solid forms of the element nitrogen, first observed in 1884. Solid nitrogen is mainly the subject of academic research, but low-temperature, low-pressure solid nitrogen is a substantial component of bodies in the outer Solar System and high-temperature, high-pressure solid nitrogen is a powerful explosive, with higher energy density than any other non-nuclear material. Generation Karol Olszewski first observed solid nitrogen in 1884, by first liquefying hydrogen with evaporating liquid nitrogen, and then allowing the liquid hydrogen to freeze the nitrogen. By evaporating vapour from the solid nitrogen, Olszewski also generated the extremely low temperature of , at the time a world record. Modern techniques usually take a similar approach: solid nitrogen is normally made in a laboratory by evaporating liquid nitrogen in a vacuum. The solid produced is porous. Occurrence in nature Solid nitrogen forms a large part of the surface of Pluto (where ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mountainous Shoreline Of Sputnik Planum (PIA20198)
A mountain is an elevated portion of the Earth's crust, generally with steep sides that show significant exposed bedrock. Although definitions vary, a mountain may differ from a plateau in having a limited summit area, and is usually higher than a hill, typically rising at least 300 metres (1,000 feet) above the surrounding land. A few mountains are isolated summits, but most occur in mountain ranges. Mountains are formed through tectonic forces, erosion, or volcanism, which act on time scales of up to tens of millions of years. Once mountain building ceases, mountains are slowly leveled through the action of weathering, through slumping and other forms of mass wasting, as well as through erosion by rivers and glaciers. High elevations on mountains produce colder climates than at sea level at similar latitude. These colder climates strongly affect the ecosystems of mountains: different elevations have different plants and animals. Because of the less hospitable terrain and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Annealing (metallurgy)
In metallurgy and materials science, annealing is a heat treatment that alters the physical and sometimes chemical properties of a material to increase its ductility and reduce its hardness, making it more workable. It involves heating a material above its recrystallization temperature, maintaining a suitable temperature for an appropriate amount of time and then cooling. In annealing, atoms migrate in the crystal lattice and the number of dislocations decreases, leading to a change in ductility and hardness. As the material cools it recrystallizes. For many alloys, including carbon steel, the crystal grain size and phase composition, which ultimately determine the material properties, are dependent on the heating rate and cooling rate. Hot working or cold working after the annealing process alters the metal structure, so further heat treatments may be used to achieve the properties required. With knowledge of the composition and phase diagram, heat treatment can be used to ad ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cubic Structure
In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals. There are three main varieties of these crystals: *Primitive cubic (abbreviated ''cP'' and alternatively called simple cubic) *Body-centered cubic (abbreviated ''cI'' or bcc) *Face-centered cubic (abbreviated ''cF'' or fcc, and alternatively called ''cubic close-packed'' or ccp) Each is subdivided into other variants listed below. Although the ''unit cells'' in these crystals are conventionally taken to be cubes, the primitive unit cells often are not. Bravais lattices The three Bravais lattices in the cubic crystal system are: The primitive cubic lattice (cP) consists of one lattice point on each corner of the cube; this means each simple cubic unit cell has in total one lattice point. Each atom at a lattice point is then shared equally between eight adjacent cubes, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
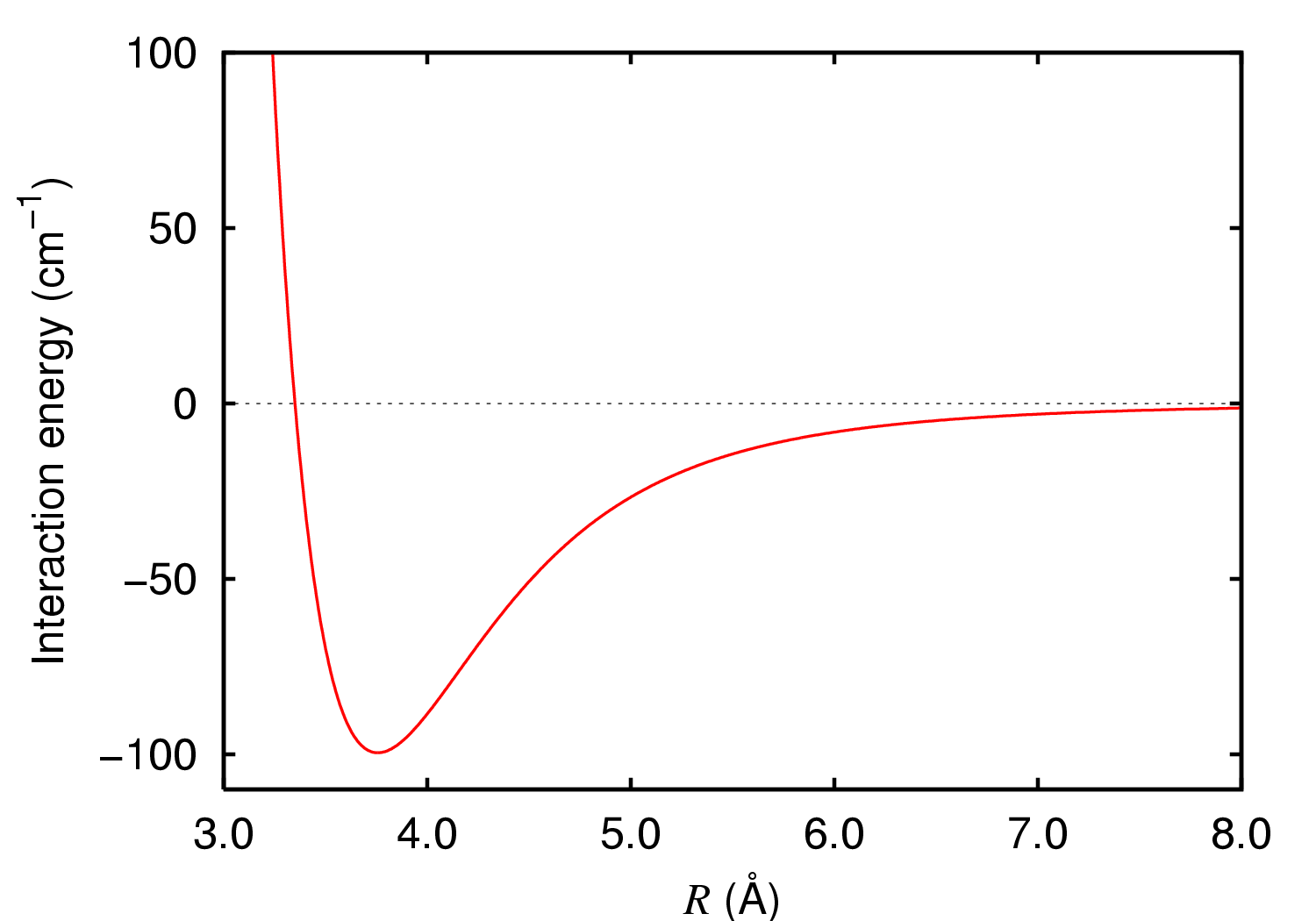
London Dispersion Force
London dispersion forces (LDF, also known as dispersion forces, London forces, instantaneous dipole–induced dipole forces, fluctuating induced dipole bonds or loosely as van der Waals forces) are a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; that is, the electrons are symmetrically distributed with respect to the nucleus. They are part of the van der Waals forces. The LDF is named after the German physicist Fritz London. They are the weakest intermolecular force. Introduction The electron distribution around an atom or molecule undergoes fluctuations in time. These fluctuations create instantaneous electric fields which are felt by other nearby atoms and molecules, which in turn adjust the spatial distribution of their own electrons. The net effect is that the fluctuations in electron positions in one atom induce a corresponding redistribution of electrons in other atoms, such that the electron motions become corre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gaseous Hydrogen
Hydrogen is the chemical element with the Symbol (chemistry), symbol H and atomic number 1. Hydrogen is the lightest element. At standard temperature and pressure, standard conditions hydrogen is a gas of diatomic molecules having the chemical formula, formula . It is transparency (optics), colorless, sense of smell, odorless, tasteless, non-toxic, and highly combustible. Hydrogen is the abundance of the chemical elements, most abundant chemical substance in the universe, constituting roughly 75% of all baryon, normal matter.However, most of the universe's mass is not in the form of baryons or chemical elements. See dark matter and dark energy. Stars such as the Sun are mainly composed of hydrogen in the plasma state. Most of the hydrogen on Earth exists in Molecular geometry, molecular forms such as water and organic compounds. For the most common isotope of hydrogen (symbol 1H) each atom has one proton, one electron, and no neutrons. In the early universe, the formation of pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Hydrogen
Liquid hydrogen (LH2 or LH2) is the liquid state of the element hydrogen. Hydrogen is found naturally in the molecular H2 form. To exist as a liquid, H2 must be cooled below its critical point of 33 K. However, for it to be in a fully liquid state at atmospheric pressure, H2 needs to be cooled to .IPTS-1968 iupac.org, accessed 2020-01-01 A common method of obtaining liquid hydrogen involves a compressor resembling a jet engine in both appearance and principle. Liquid hydrogen is typically used as a concentrated form of . Storing it as liquid takes less space than storing it as a gas at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Empirical Relationship
In science, an empirical relationship or phenomenological relationship is a relationship or correlation that is supported by experiment and observation but not necessarily supported by theory. Analytical solutions without a theory An empirical relationship is supported by confirmatory data irrespective of theoretical basis such as first principles. Sometimes theoretical explanations for what were initially empirical relationships are found, in which case the relationships are no longer considered empirical. An example was the Rydberg formula to predict the wavelengths of hydrogen spectral lines. Proposed in 1876, it perfectly predicted the wavelengths of the Lyman series, but lacked a theoretical basis until Niels Bohr produced his Bohr model of the atom in 1925.McMullin, Ernan (1968), “What Do Physical Models Tell Us?”, in B. van Rootselaar and J. F. Staal (eds.), Logic, Methodology and Science III. Amsterdam: North Holland, 385–396. On occasion, what was thought to be an em ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sublimation (phase Transition)
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state. Sublimation is an endothermic process that occurs at temperatures and pressures below a substance's triple point in its phase diagram, which corresponds to the lowest pressure at which the substance can exist as a liquid. The reverse process of sublimation is deposition or desublimation, in which a substance passes directly from a gas to a solid phase. Sublimation has also been used as a generic term to describe a solid-to-gas transition (sublimation) followed by a gas-to-solid transition ( deposition). While vaporization from liquid to gas occurs as evaporation from the surface if it occurs below the boiling point of the liquid, and as boiling with formation of bubbles in the interior of the liquid if it occurs at the boiling point, there is no such distinction for the solid-to-gas transition which always occurs as sublimation from the surface. At ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triple Point
In thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.. It is that temperature and pressure at which the sublimation curve, fusion curve and the vaporisation curve meet. For example, the triple point of mercury occurs at a temperature of and a pressure of 0.165 m Pa. In addition to the triple point for solid, liquid, and gas phases, a triple point may involve more than one solid phase, for substances with multiple polymorphs. Helium-4 is a special case that presents a triple point involving two different fluid phases (lambda point). The triple point of water was used to define the kelvin, the base unit of thermodynamic temperature in the International System of Units (SI). The value of the triple point of water was fixed by definition, rather than measured, but that changed with the 2019 redefinition of SI base units. The triple points of s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kelvin
The kelvin, symbol K, is the primary unit of temperature in the International System of Units (SI), used alongside its prefixed forms and the degree Celsius. It is named after the Belfast-born and University of Glasgow-based engineer and physicist William Thomson, 1st Baron Kelvin (1824–1907). The Kelvin scale is an absolute thermodynamic temperature scale, meaning it uses absolute zero as its null (zero) point. Historically, the Kelvin scale was developed by shifting the starting point of the much-older Celsius scale down from the melting point of water to absolute zero, and its increments still closely approximate the historic definition of a degree Celsius, but since 2019 the scale has been defined by fixing the Boltzmann constant to be exactly . Hence, one kelvin is equal to a change in the thermodynamic temperature that results in a change of thermal energy by . The temperature in degree Celsius is now defined as the temperature in kelvins minus 273.15, meaning t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gigapascal
The pascal (symbol: Pa) is the unit of pressure in the International System of Units (SI), and is also used to quantify internal pressure, stress, Young's modulus, and ultimate tensile strength. The unit, named after Blaise Pascal, is defined as one newton per square metre and is equivalent to 10 barye (Ba) in the CGS system. The unit of measurement called standard atmosphere (atm) is defined as 101,325 Pa. Common multiple units of the pascal are the hectopascal (1 hPa = 100 Pa), which is equal to one millibar, and the kilopascal (1 kPa = 1000 Pa), which is equal to one centibar. Meteorological observations typically report atmospheric pressure in hectopascals per the recommendation of the World Meteorological Organization, thus a standard atmosphere (atm) or typical sea-level air pressure is about 1013 hPa. Reports in the United States typically use inches of mercury or millibars (hectopascals). In Canada these reports are given in kilopascals. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.png)