|
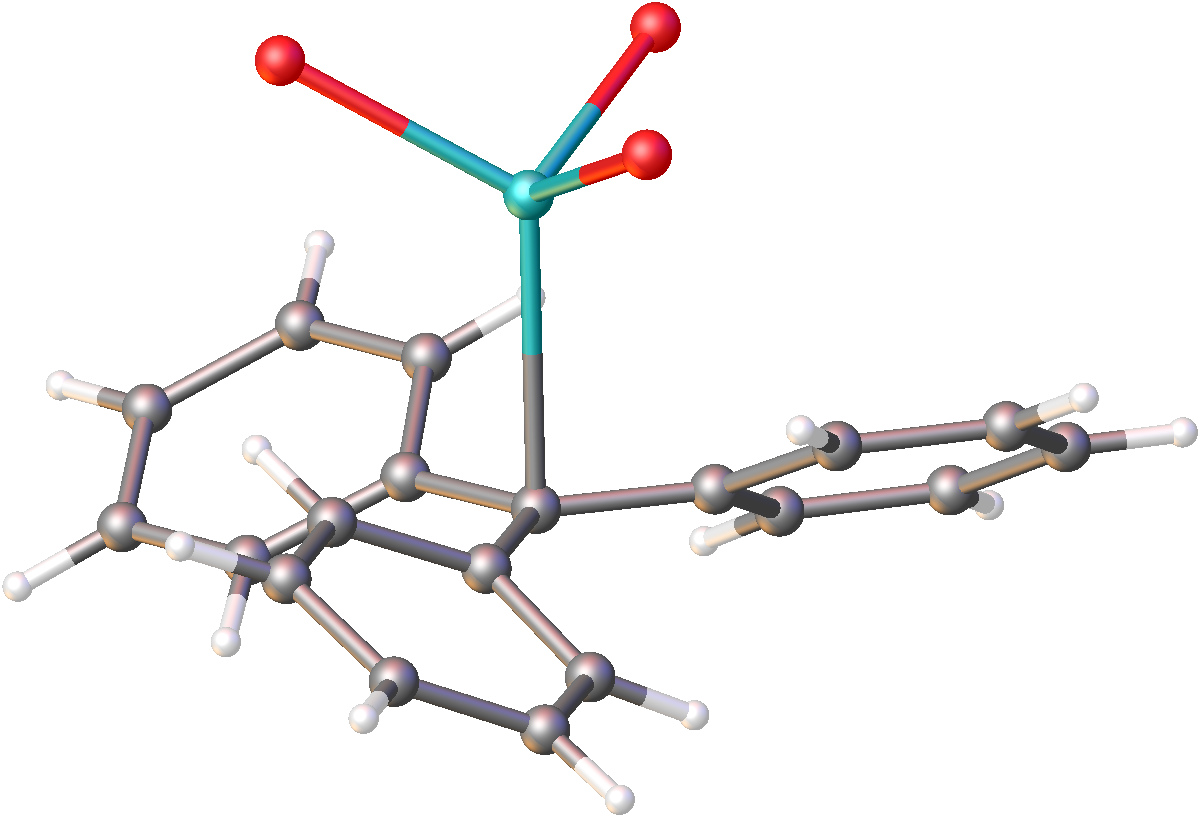
Sodium Naphthalenide
Sodium naphthalene is an organic salt with the chemical formula Na+. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. When isolated, it invariably crystallizes as a solvate with ligands bound to Na+. Preparation and properties The alkali metal naphthalene salts are prepared by stirring the metal with naphthalene in an ethereal solvent, usually as tetrahydrofuran or dimethoxyethane. The resulting salt is dark green. The anion is a radical, giving a strong EPR signal near ''g'' = 2.0. Its deep green color arises from absorptions centered at 463 and 735 nm. Several solvates of sodium naphthalenide have been characterized by X-ray crystallography. The effects are subtle, the outer pair of CH−CH bonds contract by 3 pm and the other nine C−C bonds elongate by 2–3 pm. The net effect is that reduction weakens the bonding. Reactions ;Redox Wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Naphthalene
Lithium naphthalene is an organic salt with the chemical formula Li+. In the research laboratory, it is used as a reductant in the synthesis of organic, organometallic, and inorganic chemistry. It is usually generated in situ. Lithium naphthalene crystallizes with ligands bound to Li+. Preparation and properties The compound is prepared by stirring the metallic lithium with naphthalene in an ethereal solvent, usually as tetrahydrofuran or dimethoxyethane. The resulting salt is dark green. The reaction of naphthalene with lithium can be accelerated by sonication. Methods for assaying lithium naphthalene have been developed as well. The anion is a radical, giving a strong EPR signal near ''g'' = 2.0. Its deep green color arises from absorptions at 463, 735 nm. Several solvates of lithium naphthalene have been characterized by X-ray crystallography. The effects are subtle, the outer pair of HC–CH bonds contract by 3 pm and the other nine C–C bonds elong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electron Paramagnetic Resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spins excited are those of the electrons instead of the atomic nuclei. EPR spectroscopy is particularly useful for studying metal complexes and organic radicals. EPR was first observed in Kazan State University by Soviet physicist Yevgeny Zavoisky in 1944, and was developed independently at the same time by Brebis Bleaney at the University of Oxford. Theory Origin of an EPR signal Every electron has a magnetic moment and spin quantum number s = \tfrac , with magnetic components m_\mathrm = + \tfrac or m_\mathrm = - \tfrac . In the presence of an external magnetic field with strength B_\mathrm , the electron's magnetic moment aligns itself either antiparallel ( m_\mathrm = - \tfrac ) or parallel ( m_\mathrm = + \tfrac ) to the fie ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosodium Compounds
Organosodium chemistry is the chemistry of organometallic compounds containing a carbon to sodium chemical bond. The application of organosodium compounds in chemistry is limited in part due to competition from organolithium compounds, which are commercially available and exhibit more convenient reactivity. The principal organosodium compound of commercial importance is sodium cyclopentadienide. Sodium tetraphenylborate can also be classified as an organosodium compound since in the solid state sodium is bound to the aryl groups. Organometal bonds in group 1 are characterised by high polarity with corresponding high nucleophilicity on carbon. This polarity results from the disparate electronegativity of carbon (2.55) and that of lithium 0.98, sodium 0.93 potassium 0.82 rubidium 0.82 caesium 0.79). The carbanionic nature of organosodium compounds can be minimized by resonance stabilization, for example, Ph3CNa. One consequence of the highly polarized Na-C bond is that simple org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
One-electron Reducing Agents
The one-electron universe postulate, proposed by theoretical physicist John Wheeler in a telephone call to Richard Feynman in the spring of 1940, is the hypothesis that all electrons and positrons are actually manifestations of a single entity moving backwards and forwards in time. According to Feynman: A similar "zigzag world line description of pair annihilation" has been independently devised by E. C. G. Stueckelberg at the same time. Overview The idea is based on the world lines traced out across spacetime by every electron. Rather than have myriad such lines, Wheeler suggested that they could all be parts of one single line like a huge tangled knot, traced out by the one electron. Any given moment in time is represented by a slice across spacetime, and would meet the knotted line a great many times. Each such meeting point represents a real electron at that moment. At those points, half the lines will be directed forward in time and half will have looped round and be di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dialin
Dialin (1,2-dihydronaphthalene) is a hydrocarbon with the chemical formula C10H10. It is similar to naphthalene but one ring is partially saturated. See also * Naphthalene * Tetralin * Decalin Decalin (decahydronaphthalene, also known as bicyclo .4.0ecane and sometimes decaline), a bicyclic organic compound, is an industrial solvent. A colorless liquid with an aromatic odor, it is used as a solvent for many resins or fuel additives. I ... Hydrocarbons {{hydrocarbon-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Normal Hydrogen Electrode
The standard hydrogen electrode (abbreviated SHE), is a redox electrode which forms the basis of the thermodynamic scale of oxidation-reduction potentials. Its absolute electrode potential is estimated to be at 25 °C, but to form a basis for comparison with all other electrochemical reactions, hydrogen's standard electrode potential (''E''°) is declared to be zero volts at any temperature. Potentials of all other electrodes are compared with that of the standard hydrogen electrode at the same temperature. Nernst equation for SHE The hydrogen electrode is based on the redox half cell corresponding to the reduction of two hydrated protons, 2 H+(aq), into one gaseous hydrogen molecule, H2(g). General equation for a reduction reaction: : \text + z~e^ \rightleftharpoons \text The reaction quotient (') of the half-reaction is the ratio between the chemical activities (''a'') of the reduced form (the reductant, ) and the oxidized form (the oxidant, ). : Q_r = \frac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of which are t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to diffract into many specific directions. By measuring the angles and intensities of these diffracted beams, a crystallographer can produce a three-dimensional picture of the density of electrons within the crystal. From this electron density, the mean positions of the atoms in the crystal can be determined, as well as their chemical bonds, their crystallographic disorder, and various other information. Since many materials can form crystals—such as salts, metals, minerals, semiconductors, as well as various inorganic, organic, and biological molecules—X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences among various mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is miscible with water. Production Monoglyme is produced industrially by the reaction of dimethylether with ethylene oxide: :CH3OCH3 + CH2CH2O → CH3OCH2CH2OCH3 Applications as solvent and ligand left, 144px, Structure of the coordination complex NbCl3(dimethoxyethane)(3-hexyne).{{cite journal , doi=10.1021/ja8100837, title=New Tantalum Ligand-Free Catalyst System for Highly Selective Trimerization of Ethylene Affording 1-Hexene: New Evidence of a Metallacycle Mechanism, year=2009, last1=Arteaga-Müller, first1=Rocío, last2=Tsurugi, first2=Hayato, last3=Saito, first3=Teruhiko, last4=Yanagawa, first4=Masao, last5=Oda, first5=Seiji, last6=Mashima, first6=Kazushi, journal=Journal of the American Chemical Society, volume=131, issue=15, pages=5370� ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt (chemistry)
In chemistry, a salt is a chemical compound consisting of an ionic assembly of positively charged cations and negatively charged anions, which results in a compound with no net electric charge. A common example is table salt, with positively charged sodium ions and negatively charged chloride ions. The component ions in a salt compound can be either inorganic, such as chloride (Cl−), or organic, such as acetate (). Each ion can be either monatomic, such as fluoride (F−), or polyatomic, such as sulfate (). Types of salt Salts can be classified in a variety of ways. Salts that produce hydroxide ions when dissolved in water are called ''alkali salts'' and salts that produce hydrogen ions when dissolved in water are called ''acid salts''. ''Neutral salts'' are those salts that are neither acidic nor basic. Zwitterions contain an anionic and a cationic centre in the same molecule, but are not considered salts. Examples of zwitterions are amino acids, many metabolites, peptid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is mainly used as a precursor to polymers. Being polar and having a wide liquid range, THF is a versatile solvent. Production About 200,000 tonnes of tetrahydrofuran are produced annually. The most widely used industrial process involves the acid-catalyzed dehydration of 1,4-butanediol. Ashland/ISP is one of the biggest producers of this chemical route. The method is similar to the production of diethyl ether from ethanol. The butanediol is derived from condensation of acetylene with formaldehyde followed by hydrogenation. DuPont developed a process for producing THF by oxidizing ''n''-butane to crude maleic anhydride, followed by catalytic hydrogenation. A third major industrial route entails hydroformylation of allyl alcohol followed by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl or aryl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin. Structure and bonding Ethers feature bent C–O–C linkages. In dimethyl ether, the bond angle is 111° and C–O distances are 141 pm. The barrier to rotation about the C–O bonds is low. The bonding of oxygen in ethers, alcohols, and water is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |