|
Sodium 2-hydroxyethyl Sulfonate
Sodium 2-hydroxyethyl sulfonate (also: ''sodium isethionate'') is the sodium salt of 2-hydroxyethane sulfonic acid (isethionic acid), it is used as a Hydrophile#Hydrophilic molecules, hydrophilic head group in washing-active surfactants, known as isethionates (acyloxyethanesulfonates) due to its strong polarity and resistance to multivalent ions. It is being studied as a High production volume chemicals, high production volume chemical in the "High Production Volume (HPV) Chemical Challenge Program" of the US Environmental Protection Ministry EPA. Production Sodium 2-hydroxyethyl sulfonate is formed by the reaction of ethylene oxide with sodium hydrogen sulfite in aqueous solution: : To avoid contamination and suppress the formation of by-products (which are difficult to remove) the reaction must be performed under careful control of mass ratios and process conditions. Excess sulfite (SO32−) or bisulfite (HSO3−) lead to an unpleasant odor of the downstream product, higher l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Salt
Sodium salts are salt (chemistry), salts composed of a sodium cation and the conjugate base anion of some Inorganic compound, inorganic or Organic compound, organic acids. They can be formed by the Neutralization (chemistry), neutralization of such acids with sodium hydroxide. Categorization Sodium salts can be categorized into: *sodium salts of carboxylic acids (e. g. sodium formate, HCOONa, the sodium salt of formic acid or sodium acetate, CH3COONa, the sodium salt of acetic acid, etc.) and *sodium salts of inorganic acids (Sulfonic acid, sulfonic acids etc.) Organic sodium salts Drugs In pharmaceutical technology acidic pharmaceutical substances are often converted into sodium salts, because they are more stable, more soluble or membrane-permeable (bioavailable) than the base compound. Examples of such sodium salts are (selection): Bispyribac, bithionol, bosentan, brequinar, bromfenac, Cefmenoxime, ceftiofur, citicoline, diclofenac , Flucloxacillin, Floxacillin, fosinopril ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
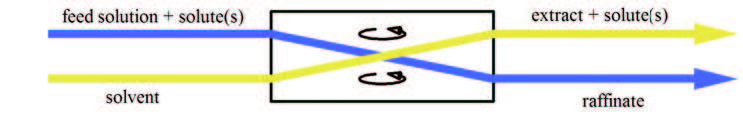
Liquid–liquid Extraction
Liquid–liquid extraction (LLE), also known as solvent extraction and partitioning, is a method to separate compounds or metal complexes, based on their relative solubilities in two different immiscible liquids, usually water (polar) and an organic solvent (non-polar). There is a net transfer of one or more species from one liquid into another liquid phase, generally from aqueous to organic. The transfer is driven by chemical potential, i.e. once the transfer is complete, the overall system of chemical components that make up the solutes and the solvents are in a more stable configuration (lower free energy). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called the raffinate. LLE is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment called as mixer settlers. This type of process is commonly performed aft ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methanesulphonic Acid
In organic chemistry, sulfonic acid (or sulphonic acid) refers to a member of the class of organosulfur compounds with the general formula , where R is an organic alkyl or aryl group and the group a sulfonyl hydroxide. As a substituent, it is known as a sulfo group. A sulfonic acid can be thought of as sulfuric acid with one hydroxyl group replaced by an organic substituent. The parent compound (with the organic substituent replaced by hydrogen) is the parent sulfonic acid, , a tautomer of sulfurous acid, . Salt (chemistry), Salts or esters of sulfonic acids are called sulfonates. Preparation Aryl sulfonic acids are produced by the process of sulfonation. Usually the sulfonating agent is sulfur trioxide. A large scale application of this method is the production of alkylbenzenesulfonic acids: :RC6H5 + SO3 -> RC6H4SO3H In this reaction, sulfur trioxide is an electrophile and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkyl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |