|
Soai Reaction
In organic chemistry, the Soai reaction is the alkylation of pyrimidine-5-carbaldehyde with diisopropylzinc. The reaction is autocatalytic and leads to rapidly increasing amounts of the same enantiomer of the product. The product pyrimidyl alcohol is chiral and induces that same chirality in further catalytic cycles. Starting with a low enantiomeric excess produces a product with very high enantiomeric excess. The reaction has been studied for clues about the origin of homochirality among certain classes of biomolecules. : The Japanese chemist Kensō Soai (1950–) discovered the reaction in 1995. For his work in "elucidating the origins of chirality and homochirality", Soai received the Chemical Society of Japan award in 2010. Other chiral additives can be used as the initial source of asymmetric induction, with the major product of that first reaction being rapidly amplified. For example, Soai's group has demonstrated that even chiral quaternary hydrocarbons, which have no clear ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkylation
Alkylation is the transfer of an alkyl group from one molecule to another. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents. Nucleophilic alkylating agents Nucleophilic alkylating agents deliver the equivalent of an alkyl anion ( carbanion). The formal "alkyl anion" attacks an electrophile, forming a new covalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
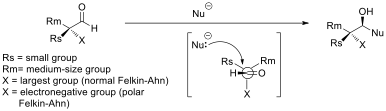
Asymmetric Induction
In stereochemistry, asymmetric induction (also enantioinduction) describes the preferential formation in a chemical reaction of one enantiomer or diastereoisomer over the other as a result of the influence of a chiral feature present in the substrate, reagent, catalyst or environment. Asymmetric induction is a key element in asymmetric synthesis. Asymmetric induction was introduced by Hermann Emil Fischer based on his work on carbohydrates. Several types of induction exist. Internal asymmetric induction makes use of a chiral center bound to the reactive center through a covalent bond and remains so during the reaction. The starting material is often derived from chiral pool synthesis. In relayed asymmetric induction the chiral information is introduced in a separate step and removed again in a separate chemical reaction. Special synthons are called chiral auxiliaries. In external asymmetric induction chiral information is introduced in the transition state through a catalyst o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stereochemistry
Stereochemistry, a subdiscipline of chemistry, involves the study of the relative spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which by definition have the same molecular formula and sequence of bonded atoms (constitution), but differ in structural formula (the three-dimensional orientations of their atoms in space). For this reason, it is also known as 3D chemistry—the prefix "stereo-" means "three-dimensionality". Stereochemistry spans the entire spectrum of organic, inorganic, biological, physical and especially supramolecular chemistry. Stereochemistry includes methods for determining and describing these relationships; the effect on the physical or biological properties these relationships impart upon the molecules in question, and the manner in which these relationships influence the reactivity of the molecules in question ( dynamic stereochemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pi Electron
In chemistry, pi bonds (π bonds) are covalent chemical bonds, in each of which two lobes of an orbital on one atom overlap with two lobes of an orbital on another atom, and in which this overlap occurs laterally. Each of these atomic orbitals has an electron density of zero at a shared nodal plane that passes through the two bonded nuclei. This plane also is a nodal plane for the molecular orbital of the pi bond. Pi bonds can form in double and triple bonds but do not form in single bonds in most cases. The Greek letter π in their name refers to p orbitals, since the orbital symmetry of the pi bond is the same as that of the p orbital when seen down the bond axis. One common form of this sort of bonding involves p orbitals themselves, though d orbitals also engage in pi bonding. This latter mode forms part of the basis for metal-metal multiple bonding. Pi bonds are usually weaker than sigma bonds. The C-C double bond, composed of one sigma and one pi bond, has a bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Basic
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane (Me3B) is a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as posse ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quaternary Compound
In chemistry, a quaternary compound is a compound consisting of exactly four chemical elements. In another use of the term in organic chemistry, a quaternary compound is or has a cation consisting of a central positively charged atom with four substituents, especially organic (alkyl and aryl) groups, discounting hydrogen atoms. The best-known quaternary compounds are quaternary ammonium salts, having a nitrogen atom at the centre. For example, in the following reaction, the nitrogen atom is said to be quaternized as it has gone from 3 to 4 substituents: :R3N + RCl -> R4N+Cl- Other examples include substituted phosphonium salts (), substituted arsonium salts () like arsenobetaine, as well as some arsenic-containing superconductors. Substituted stibonium () and bismuthonium salts () have also been described. See also *Binary compound *Ternary compound *Onium ion *Quaternary phase In materials chemistry, a quaternary phase is a chemical compound containing four element ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Society Of Japan
The (CSJ) is a learned society and professional association founded in 1878 in order to advance research in chemistry. The mission of the CSJ is to promote chemistry for science and industry in collaboration with other domestic and global societies.Chemical Society of Japan (CSJ) About CSJ/ref> History The organization was modeled after the British Chemical Society. This learned society in London was the precursor of the Royal Society of Chemistry. Like its British counterpart, the Japanese association sought to foster the communication of new ideas and facts throughout Japan and across international borders.Lagowski, J. J. (1991) "A British Sesquicentennial,"''Journal of Chemical Education,'' Vol 68, No. 1, p. 1; acknowledging the sesquicentennial of The Chemical Society in London, which eventually became The Royal Society of Chemistry. Membership was expanded in 1948 in a merger with the Society of Chemical Industry. In 2018 the first woman was announced as president, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diisopropylzinc
Diisopropylzinc is an organozinc compound with the chemical formula ZnC6H14. It is the key reagent in the Soai reaction, which is both autocatalytic and enantiospecific. This chemical is pyrophoric A substance is pyrophoric (from grc-gre, πυροφόρος, , 'fire-bearing') if it ignites spontaneously in air at or below (for gases) or within 5 minutes after coming into contact with air (for liquids and solids). Examples are organolit ..., bursting into flame in air or in contact with water. It is generally packaged in toluene. References Organozinc compounds Isopropyl compounds {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kensō Soai
(born 1950) is a Japanese organic chemist. He is a university lecturer in the Applied Chemistry Department of Tokyo University of Science. Soai studied at the University of Tokyo, where he received his Ph.D. in 1979 in organic synthesis under Teruaki Mukaiyama and was a fellow of the Japan Society for the Promotion of Science. He conducted his postdoctoral studies with Ernest L. Eliel at the University of North Carolina. In 1981, he became a lecturer at Tokyo University of Science, and was promoted to associate professor (1986) and full professor (1991). Official university website He is involved in asymmetric and , asymmetric |