|
Simes Procedure
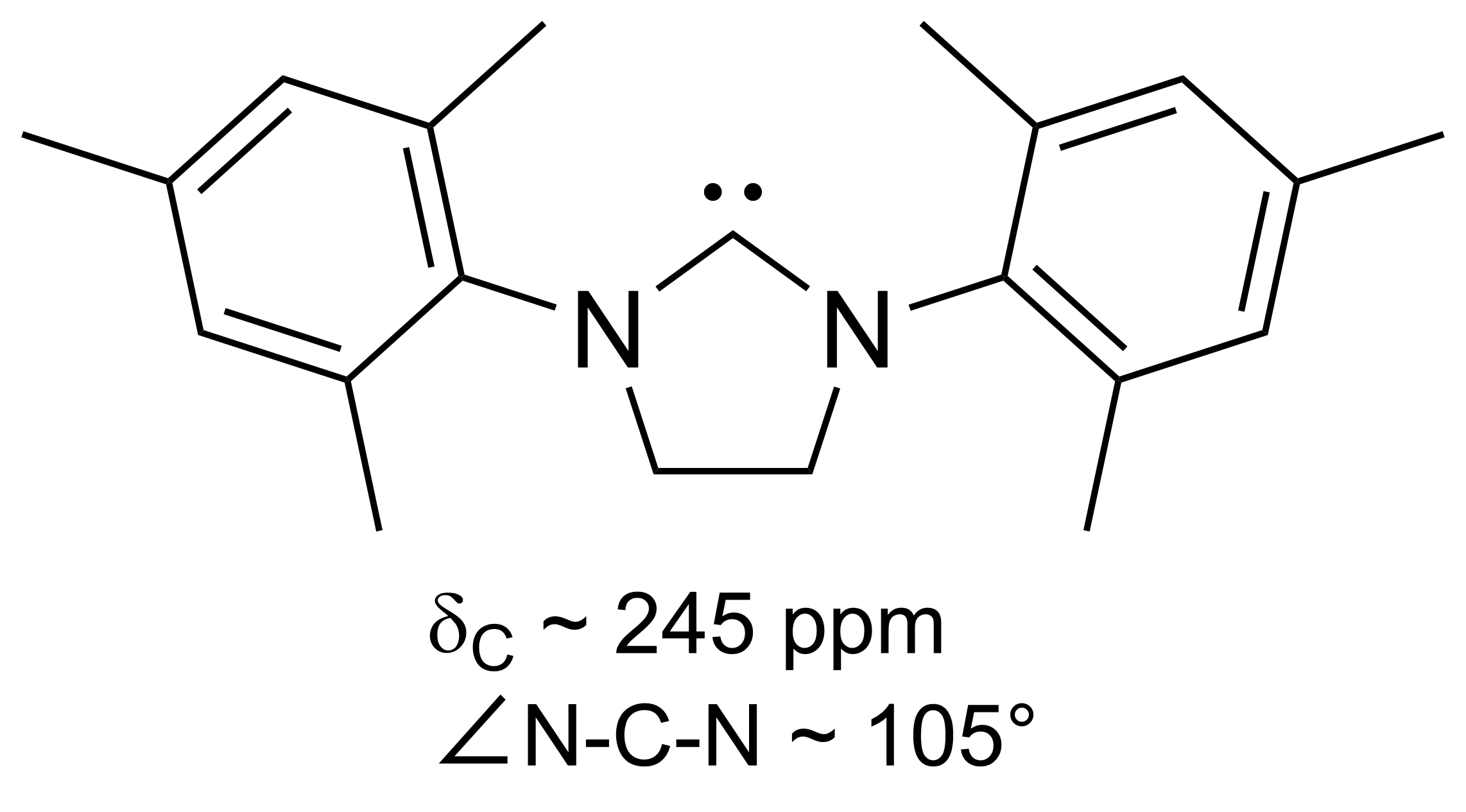
SIMes (or H2Imes) is an ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and so .... It is structurally related to the more common ligand IMes but with a saturated backbone (the S of SIMes indicates a saturated backbone). It is slightly more flexible and is a component in Grubbs II. It is prepared by alkylation of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation. References {{organic-compound-stub Carbenes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-heterocyclic Carbene
A persistent carbene (also known as stable carbene) is a type of carbene demonstrating particular stability. The best-known examples and by far largest subgroup are the ''N''-heterocyclic carbenes (NHC) (sometimes called Arduengo carbenes), for example diaminocarbenes with the general formula (R2N)2C:, where the four R moieties are typically alkyl and aryl groups. The groups can be linked to give heterocyclic carbenes, such as those derived from imidazole, imidazoline, thiazole or triazole. Traditionally carbenes are viewed as so reactive that were only studied indirectly, such as by trapping reactions. This situation has changed dramatically with the emergence of persistent carbenes. Although they are fairly reactive substances, undergoing dimerization, many can be isolated as pure substances. Persistent carbenes tend to exist in the singlet. Their stability is only partly due to steric hindrance by bulky groups. Some singlet carbenes are thermodynamically stable and can be iso ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term " metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal phosphine complexes are repres ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grubbs' Catalyst
Grubbs catalysts are a series of transition metal carbene complexes used as catalysts for olefin metathesis. They are named after Robert H. Grubbs, the chemist who supervised their synthesis. Several generations of the catalyst have been developed. Grubbs catalysts tolerate many functional groups in the alkene substrates, are air-tolerant, and are compatible with a wide range of solvents. For these reasons, Grubbs catalysts have become popular in synthetic organic chemistry. Grubbs, together with Richard R. Schrock and Yves Chauvin, won the Nobel Prize in Chemistry in recognition of their contributions to the development of olefin metathesis. First-generation catalyst In the 1960s, ruthenium trichloride was found to catalyze olefin metathesis. Processes were commercialized based on these discoveries. These ill-defined but highly active homogeneous catalysts remain in industrial use. The first well-defined ruthenium catalyst was reported in 1992. It was prepared from RuCl2(PPh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |