|
SHP-2
Tyrosine-protein phosphatase non-receptor type 11 (PTPN11) also known as protein-tyrosine phosphatase 1D (PTP-1D), Src homology region 2 domain-containing phosphatase-2 (SHP-2), or protein-tyrosine phosphatase 2C (PTP-2C) is an enzyme that in humans is encoded by the ''PTPN11'' gene. PTPN11 is a protein tyrosine phosphatase (PTP) Shp2. PTPN11 is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. This PTP contains two tandem Src homology-2 domains, which function as phospho-tyrosine binding domains and mediate the interaction of this PTP with its substrates. This PTP is widely expressed in most tissues and plays a regulatory role in various cell signaling events that are important for a diversity of cell functions, such as mitogenic activation, metabolic control, transcription regulation, and cell migratio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
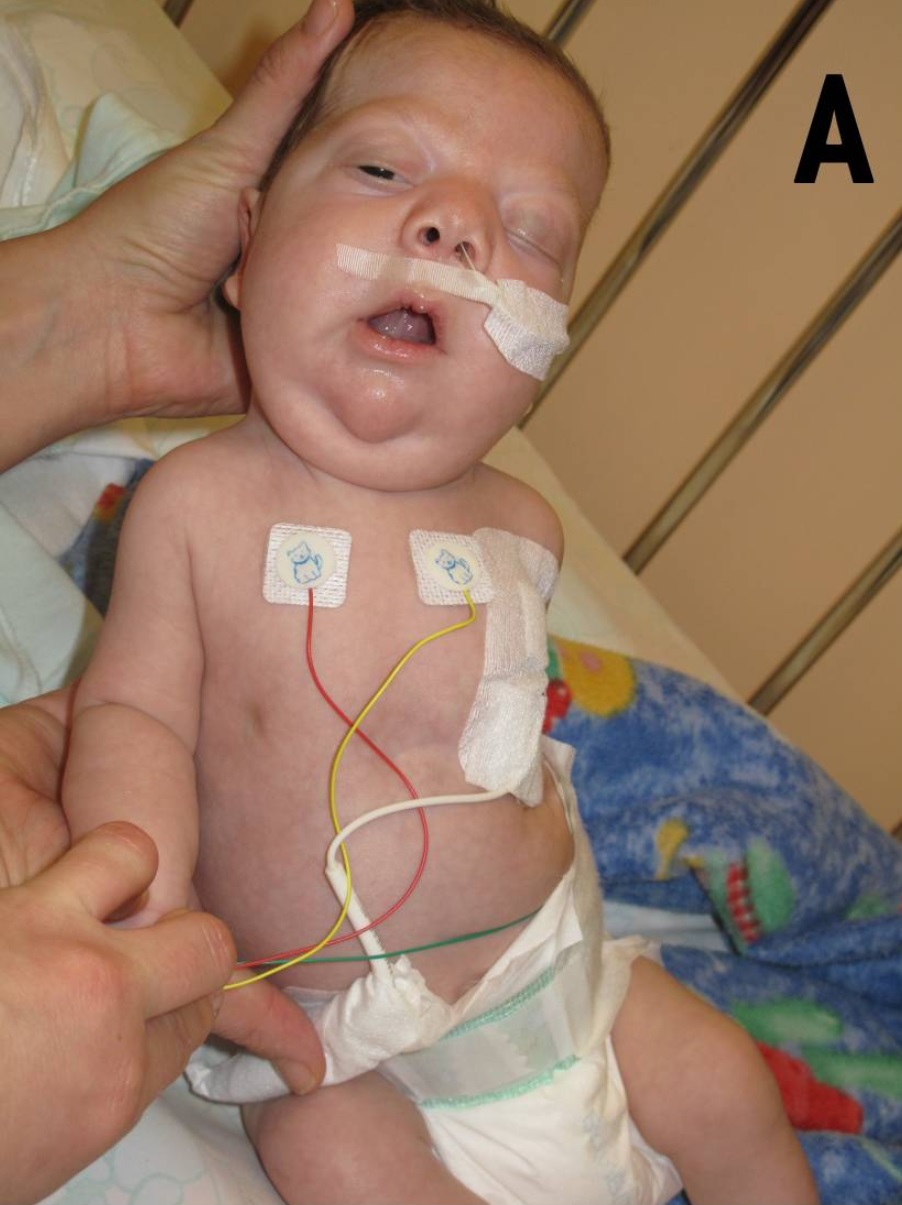
Noonan Syndrome
Noonan syndrome (NS) is a genetic disorder that may present with mildly unusual facial features, short height, congenital heart disease, bleeding problems, and skeletal malformations. Facial features include widely spaced eyes, light-colored eyes, low-set ears, a short neck, and a small lower jaw. Heart problems may include pulmonary valve stenosis. The breast bone may either protrude or be sunken, while the spine may be abnormally curved. Intelligence in the syndrome is often normal. Complications of NS can include leukemia. A number of genetic mutations can result in Noonan syndrome. The condition may be inherited from a person's parents as an autosomal dominant condition or occur as a new mutation. Noonan syndrome is a type of RASopathy, the underlying mechanism for which involves overactivation within the RAS/MAPK cell signaling pathway. The diagnosis may be suspected based on symptoms, medical imaging, and blood tests. Confirmation may be achieved with genetic tes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Shp1
Tyrosine-protein phosphatase non-receptor type 6, also known as Src homology region 2 domain-containing phosphatase-1 (SHP-1), is an enzyme that in humans is encoded by the ''PTPN6'' gene. Function The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family. PTPs are known to be signaling molecules that regulate a variety of cellular processes including cell growth, differentiation, mitotic cycle, and oncogenic transformation. N-terminal part of this PTP contains two tandem Src homolog ( SH2) domains, which act as protein phospho-tyrosine binding domains, and mediate the interaction of this PTP with its substrates. This PTP is expressed primarily in hematopoietic cells, and functions as an important regulator of multiple signaling pathways in hematopoietic cells. This PTP has been shown to interact with, and dephosphorylate a wide spectrum of phospho-proteins involved in hematopoietic cell signaling, (e.g., the LYN-CD22-SHP-1 pathway). Multiple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CEACAM1
Carcinoembryonic antigen-related cell adhesion molecule 1 (biliary glycoprotein) (CEACAM1) also known as CD66a (Cluster of Differentiation 66a), is a human glycoprotein, and a member of the carcinoembryonic antigen (CEA) gene family. Function This gene encodes a member of the carcinoembryonic antigen (CEA) gene family, which belongs to the immunoglobulin superfamily. Two subgroups of the CEA family, the CEA cell adhesion molecules and the pregnancy-specific glycoproteins, are located within a 1.2 Mb cluster on the long arm of chromosome 19. Eleven pseudogenes of the CEA cell adhesion molecule subgroup are also found in the cluster. The encoded protein was originally described in bile ducts of liver as biliary glycoprotein. Subsequently, it was found to be a cell–cell adhesion molecule detected on leukocytes, epithelia, and endothelia. The encoded protein mediates cell adhesion via homophilic as well as heterophilic binding to other proteins of the subgroup. Multiple cellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Metachondromatosis
Metachondromatosis is an autosomal dominant, incompletely penetrant genetic disease affecting the growth of bones, leading to exostoses primarily in the hands and feet as well as enchondromas of long bone metaphyses and iliac crests. This syndrome affects mainly tubular bones, though it can also involve the vertebrae, small joints, and flat bones. The disease is thought to affect exon 4 of the PTPN11 gene. Metachondromatosis is believed to be caused by an 11 base pair deletion resulting in a frameshift and nonsense mutation. The disease was discovered and named in 1971 by Pierre Maroteaux, a French physician, when he observed two families with skeletal radiologic features with exostoses and Ollier disease. The observation of one family with five affected people led to the identification of the disease as autosomal dominant. There have been less than 40 cases of the disease reported to date. Signs and Symptoms Metachondromatosis is identified by the presence of both multiple ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CD31
Platelet endothelial cell adhesion molecule (PECAM-1) also known as cluster of differentiation 31 (CD31) is a protein that in humans is encoded by the ''PECAM1'' gene found on chromosome17q23.3. PECAM-1 plays a key role in removing aged neutrophils from the body. Structure PECAM-1 is a highly glycosylated protein with a mass of approximately 130 kDa. The structure of this protein was determined by molecular cloning in 1990, when it was found out that PECAM-1 has N-terminal domain with 574 amino acids, transmembrane domain with 19 amino acids and C-terminal cytoplasmic domain with 118 amino acids. The N-terminal domain consists of six extracellular Ig-like domains. Interactions PECAM-1 is a cell-cell adhesion protein which interacts with other PECAM-1 molecules through homophilic interactions or with non-PECAM-1 molecules through heterophilic interactions''.'' Homophilic interactions between PECAM-1 molecules are mediated by antiparallel interactions between extracellular ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CagA
''Helicobacter pylori'' virulence factor CagA (cytotoxin-associated gene A) is a 120–145kDa protein encoded on the 40kb ''cag'' pathogenicity island (PAI). ''H. pylori'' strains can be divided into CagA positive or negative strains. Approximately 60% of ''H. pylori'' strains isolated in Western countries carry ''cag'' PAI, whereas almost all of the East Asian isolates are ''cag'' PAI-positive. The ''cag'' PAI also encodes for a type IV secretion system which is used to "inject" CagA into a target cell upon ''H. pylori'' attachment. After translocation, CagA localises to the inner surface of the cell membrane and undergoes tyrosine phosphorylation by Src family kinases (e.g. Fyn and Lyn). Role in Cancer ''H. pylori'' infection is associated with MALT lymphoma and gastric adenocarcinoma and CagA is thought to be involved in cancer development. Phosphorylated CagA is able to interact with the SHP-2 tyrosine phosphatase, rendering it functionally active, triggering a host cell morp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leopard Syndrome
Noonan syndrome with multiple lentigines (NSML) which is part of a group called Ras/MAPK pathway syndromes, is a rare autosomal dominant, multisystem disease caused by a mutation in the protein tyrosine phosphatase, non-receptor type 11 gene (''PTPN11''). The disease is a complex of features, mostly involving the skin, skeletal and cardiovascular systems, which may or may not be present in all patients. The nature of how the mutation causes each of the condition's symptoms is not well known; however, research is ongoing. It is a RASopathy. Noonan syndrome with multiple lentigines is caused by a different missense mutation of the same gene. Noonan syndrome is fairly common (1:1,000 to 1:2,500 live births), and neurofibromatosis 1 (which was once thought to be related to NSML) is also common (1:3500); however, no epidemiological data exists for NSML. Signs and symptoms An alternative name of the condition, LEOPARD syndrome, is a mnemonic, originally coined in 1969, as the condition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cbl Gene
''Cbl'' (named after Casitas B-lineage Lymphoma) is a mammalian gene encoding the protein CBL which is an E3 ubiquitin-protein ligase involved in cell signalling and protein ubiquitination. Mutations to this gene have been implicated in a number of human cancers, particularly acute myeloid leukaemia. Discovery In 1989 a virally encoded portion of the chromosomal mouse ''Cbl'' gene was the first member of the Cbl family to be discovered and was named ''v-Cbl'' to distinguish it from normal mouse ''c-Cbl''. The virus used in the experiment was a mouse-tropic strain of Murine leukemia virus isolated from the brain of a mouse captured at Lake Casitas, California known as ''Cas-Br-M'', and was found to have excised approximately a third of the original ''c-Cbl'' gene from a mouse into which it was injected. Sequencing revealed that the portion carried by the retrovirus encoded a ''tyrosine kinase binding domain'', and that this was the oncogenic form as retroviruses carrying ful ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Necrosis
Necrosis () is a form of cell injury which results in the premature death of cells in living tissue by autolysis. Necrosis is caused by factors external to the cell or tissue, such as infection, or trauma which result in the unregulated digestion of cell components. In contrast, apoptosis is a naturally occurring programmed and targeted cause of cellular death. While apoptosis often provides beneficial effects to the organism, necrosis is almost always detrimental and can be fatal. Cellular death due to necrosis does not follow the apoptotic signal transduction pathway, but rather various receptors are activated and result in the loss of cell membrane integrity and an uncontrolled release of products of cell death into the extracellular space. This initiates in the surrounding tissue an inflammatory response, which attracts leukocytes and nearby phagocytes which eliminate the dead cells by phagocytosis. However, microbial damaging substances released by leukocytes would crea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatocellular Carcinoma
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide. It occurs in the setting of chronic liver inflammation, and is most closely linked to chronic viral hepatitis infection (hepatitis B or C) or exposure to toxins such as alcohol, aflatoxin, or pyrrolizidine alkaloids. Certain diseases, such as hemochromatosis and alpha 1-antitrypsin deficiency, markedly increase the risk of developing HCC. Metabolic syndrome and NASH are also increasingly recognized as risk factors for HCC. As with any cancer, the treatment and prognosis of HCC vary depending on the specifics of tumor histology, size, how far the cancer has spread, and overall health. The vast majority of HCC cases and the lowest survival rates after treatment occur in Asia and sub-Saharan Africa, in countries where hepatitis B infection is endem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Helicobacter Pylori
''Helicobacter pylori'', previously known as ''Campylobacter pylori'', is a gram-negative, microaerophilic, spiral (helical) bacterium usually found in the stomach. Its helical shape (from which the genus name, helicobacter, derives) is thought to have evolved in order to penetrate the mucoid lining of the stomach and thereby establish infection. The bacterium was first identified in 1982 by the Australian doctors Barry Marshall and Robin Warren. ''H. pylori'' has been associated with cancer of the mucosa-associated lymphoid tissue in the stomach, esophagus, colon, rectum, or tissues around the eye (termed extranodal marginal zone B-cell lymphoma of the cited organ), and of lymphoid tissue in the stomach (termed diffuse large B-cell lymphoma). ''H. pylori'' infection usually has no symptoms but sometimes causes gastritis (stomach inflammation) or ulcers of the stomach or first part of the small intestine. The infection is also associated with the development of cer ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
Enzymes () are proteins that act as biological catalysts by accelerating chemical reactions. The molecules upon which enzymes may act are called substrates, and the enzyme converts the substrates into different molecules known as products. Almost all metabolic processes in the cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts are catalytic RNA molecules, called ribozymes. Enzymes' specificity comes from their unique three-dimensional structures. Like all catalysts, enzymes increase the reaction ra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |