|
Shaker (gene)
The ''shaker (Sh)'' gene, when mutated, causes a variety of atypical behaviors in the fruit fly, ''Drosophila melanogaster''. Under ether anesthesia, the fly’s legs will shake (hence the name); even when the fly is unanaesthetized, it will exhibit aberrant movements. Sh-mutant flies have a shorter lifespan than regular flies; in their larvae, the repetitive firing of action potentials as well as prolonged exposure to neurotransmitters at neuromuscular junctions occurs. In ''Drosophila'', the shaker gene is located on the X chromosome. The closest human homolog is KCNA3. Function The ''Sh'' gene plays a part in the operation of potassium ion channels, which are integral membrane proteins and are essential to the correct functioning of the cell. A working shaker channel is voltage-dependent and has four subunits, which form a pore through which ions flow, carrying type-A potassium current (IA). A mutation in the Sh gene reduces the conductance of charge across the neuron s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
KCNA3
Potassium voltage-gated channel, shaker-related subfamily, member 3, also known as KCNA3 or Kv1.3, is a protein that in humans is encoded by the ''KCNA3'' gene. Potassium channels represent the most complex class of voltage-gated ion channels from both functional and structural standpoints. Their diverse functions include regulating neurotransmitter release, heart rate, insulin secretion, neuronal excitability, epithelial electrolyte transport, smooth muscle contraction, and cell volume. Four sequence-related potassium channel genes – shaker, shaw, shab, and shal – have been identified in Drosophila, and each has been shown to have human homolog(s). This gene encodes a member of the potassium channel, voltage-gated, shaker-related subfamily. This member contains six membrane-spanning domains with a shaker-type repeat in the fourth segment. It belongs to the delayed rectifier class, members of which allow nerve cells to efficiently repolarize following an action potential. It ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
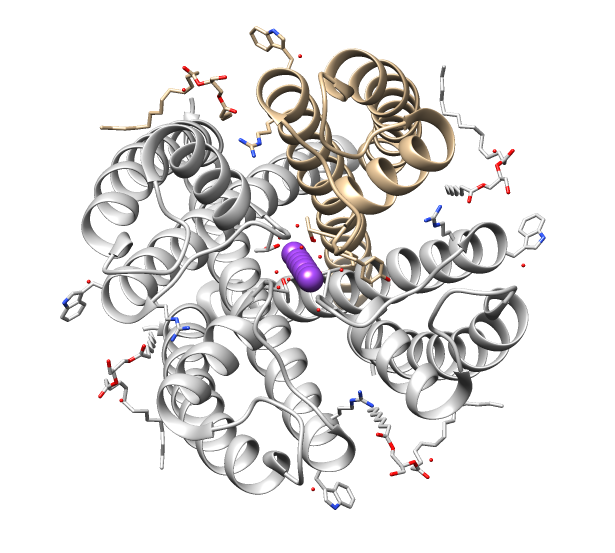
Ion Channels
Ion channels are pore-forming membrane proteins that allow ions to pass through the channel pore. Their functions include establishing a resting membrane potential, shaping action potentials and other electrical signals by gating the flow of ions across the cell membrane, controlling the flow of ions across secretory and epithelial cells, and regulating cell volume. Ion channels are present in the membranes of all cells. Ion channels are one of the two classes of ionophoric proteins, the other being ion transporters. The study of ion channels often involves biophysics, electrophysiology, and pharmacology, while using techniques including voltage clamp, patch clamp, immunohistochemistry, X-ray crystallography, fluoroscopy, and RT-PCR. Their classification as molecules is referred to as channelomics. Basic features There are two distinctive features of ion channels that differentiate them from other types of ion transporter proteins: #The rate of ion transport through the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drosophila Melanogaster Genes
''Drosophila'' () is a genus of flies, belonging to the family Drosophilidae, whose members are often called "small fruit flies" or (less frequently) pomace flies, vinegar flies, or wine flies, a reference to the characteristic of many species to linger around overripe or rotting fruit. They should not be confused with the Tephritidae, a related family, which are also called fruit flies (sometimes referred to as "true fruit flies"); tephritids feed primarily on unripe or ripe fruit, with many species being regarded as destructive agricultural pests, especially the Mediterranean fruit fly. One species of ''Drosophila'' in particular, ''D. melanogaster'', has been heavily used in research in genetics and is a common model organism in developmental biology. The terms "fruit fly" and "''Drosophila''" are often used synonymously with ''D. melanogaster'' in modern biological literature. The entire genus, however, contains more than 1,500 species and is very diverse in appearance, beh ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genetically Encoded Voltage Indicator
Genetically encoded voltage indicator (or GEVI) is a protein that can sense membrane potential in a cell and relate the change in voltage to a form of output, often fluorescent level. It is a promising optogenetic recording tool that enables exporting electrophysiological signals from cultured cells, live animals, and ultimately human brain. Examples of notable GEVIs include ArcLight, ASAP1, ASAP3, Archons, SomArchon, and Ace2N-mNeon. History Despite that the idea of optical measurement of neuronal activity was proposed in the late 1960s, the first successful GEVI that was convenient enough to put into actual use was not developed until technologies of genetic engineering had become mature in the late 1990s. The first GEVI, coined FlaSh, was constructed by fusing a modified green fluorescent protein with a voltage-sensitive K+ channel ( Shaker). Unlike fluorescent proteins, the discovery of new GEVIs were seldom inspired by the nature, for it is hard to find an organism which ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pandinotoxin
Pandinotoxins are toxins from the venom of the emperor scorpion '' Pandinus imperator''. They are selective blockers of voltage-gated potassium channels Sources The source for the pandinotoxins is the venom of the scorpion '' Pandinus imperator''. Chemistry Family The toxins of the family are designated pandinotoxin (PiTX)-Kα, PiTX-Kβ, and PiTX-Kγ They are members of the α-KTx family of scorpion toxins. Structure and homology Pandinotoxin Kα and -β The amino acid sequences of PiTX-K α and PiTX-K β are identical, except for the seventh amino acid: a proline in PiTX-Kα and a glutamic acid in PiTX-Kβ (see Fig.1). PiTX-Kα and PiTX-Kβ are 35-residue peptides, which are found to have an α-helix from residues 10 to 21 and two β-sheets (β 1 is from residues 26-28, β 2 is from residues 33-35). One face of the α-helix is anchored to the β-sheet by three disulfide bonds which are conserved in all members of the charybdotoxin family (R-K toxins). PiTX-K α and PiT ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iberiotoxin
Iberiotoxin (IbTX) is an ion channel toxin purified from the Eastern Indian red scorpion ''Hottentotta tamulus''. Iberiotoxin selectively inhibits the current through large-conductance calcium-activated potassium channels. Chemistry Iberiotoxin is a 37-amino acid peptide. The formula is C179H274N50O55S7. It is also known as "Potassium channel toxin alpha-KTx 1.3" or IbTx. The complete amino acid sequence has been defined and it displays 68% sequence homology with charybdotoxin. Target and mode of action Iberiotoxin binds to the outer face of the large-conductance calcium-activated potassium channels (maxiK or BK channels) with high affinity (Kd ~1 nM). It selectively inhibits the current by decreasing both the probability of opening and the open time of the channel. Toxicity The venom produces mainly cardiopulmonary abnormalities like circulatory derangements, myocarditis and changes in cardiac sarcolemmal ATPase and by these abnormalities it can finally cause death. In rur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charybdotoxin
Charybdotoxin (CTX) is a 37 amino acid neurotoxin from the venom of the scorpion '' Leiurus quinquestriatus hebraeus'' (''deathstalker'') that blocks calcium-activated potassium channels. This blockade causes hyperexcitability of the nervous system. It is a close homologue of agitoxin and both toxins come from ''Leiurus quinquestriatus hebraeus''. It is named after Charybdis, a sea monster from Greek myth. Chemical properties Family The Charybdotoxin family of scorpion toxins is a group of small peptides that has many family members, such as the pandinotoxin, derived from the venom of scorpion Pandinus imperator. Structure Scorpions such as the deathstalker paralyze their prey by injecting a potent mix of peptide toxins. Charybdotoxin, a 37 amino acid, 4 kDa neurotoxin with the molecular formula C176H277N57O55S7, is one of the peptide toxins that can be extracted from the venom of the scorpion. Its structure is very similar to that of margatoxin. Charybdotoxin contains three d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agitoxin
Agitoxin is a toxin found in the venom of the scorpion '' Leiurus quinquestriatus hebraeus'' (yellow scorpion). Other toxins found in this species include charybdotoxin (CTX). CTX is a close homologue of Agitoxin. Structure Agitoxin can be purified using HPLC techniques. Primary structure: Three types of agitoxin can be distinguished, each identified as comprising 38 amino acids. They are highly homologous, differing only in the identity of the residues at positions 7, 15, 29 and 31. *Agitoxin-1 Gly-Val-Pro-Ile-Asn-Val-Lys-Cys-Thr-Gly-Ser-Pro-Gln-Cys-Leu-Lys-Pro-Cys-Lys-Asp-Ala-Gly-Met-Arg-Phe-Gly-Lys-Cys-Ile-Asn-Gly-Lys-Cys-His-Cys-Thr-Pro-Lys (GVPINVKCTGSPQCLKPCKDAGMRFGKCINGKCHCTPK, molecular weight = 4014.87 Da, molecular formula = C169H278N52O47S7) *Agitoxin-2 Gly-Val-Pro-Ile-Asn-Val-Ser-Cys-Thr-Gly-Ser-Pro-Gln-Cys-Ile-Lys-Pro-Cys-Lys-Asp-Ala-Gly-Met-Arg-Phe-Gly-Lys-Cys-Met-Asn-Arg-Lys-Cys-His-Cys-Thr-Pro-Lys (GVPINVSCTGSPQCIKPCKDAGMRFGKCMNRKCHCTPK, molecular weight ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetramer
A tetramer () (''tetra-'', "four" + '' -mer'', "parts") is an oligomer formed from four monomers or subunits. The associated property is called ''tetramery''. An example from inorganic chemistry is titanium methoxide with the empirical formula Ti(OCH3)4, which is tetrameric in solid state and has the molecular formula Ti4(OCH3)16. An example from organic chemistry is kobophenol A, a substance that is formed by combining four molecules of resveratrol. In biochemistry, it similarly refers to a biomolecule formed of four units, that are the same (homotetramer), i.e. as in Concanavalin A or different (heterotetramer), i.e. as in hemoglobin. Hemoglobin has 4 similar sub-units while immunoglobulins have 2 very different sub-units. The different sub-units may have each their own activity, such as binding biotin in avidin tetramers, or have a common biological property, such as the allosteric binding of oxygen in hemoglobin. See also * Cluster chemistry; atomic and molecular clusters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Repolarization
In neuroscience, repolarization refers to the change in membrane potential that returns it to a negative value just after the depolarization phase of an action potential which has changed the membrane potential to a positive value. The repolarization phase usually returns the membrane potential back to the resting membrane potential. The efflux of potassium (K+) ions results in the falling phase of an action potential. The ions pass through the selectivity filter of the K+ channel pore. Repolarization typically results from the movement of positively charged K+ ions out of the cell. The repolarization phase of an action potential initially results in hyperpolarization, attainment of a membrane potential, termed the afterhyperpolarization, that is more negative than the resting potential. Repolarization usually takes several milliseconds. Repolarization is a stage of an action potential in which the cell experiences a decrease of voltage due to the efflux of potassium (K+) ions ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Voltage-gated Ion Channel
Voltage-gated ion channels are a class of transmembrane proteins that form ion channels that are activated by changes in the electrical membrane potential near the channel. The membrane potential alters the conformation of the channel proteins, regulating their opening and closing. Cell membranes are generally impermeable to ions, thus they must diffuse through the membrane through transmembrane protein channels. They have a crucial role in excitable cells such as neuronal and muscle tissues, allowing a rapid and co-ordinated depolarization in response to triggering voltage change. Found along the axon and at the synapse, voltage-gated ion channels directionally propagate electrical signals. Voltage-gated ion-channels are usually ion-specific, and channels specific to sodium (Na+), potassium (K+), calcium (Ca2+), and chloride (Cl−) ions have been identified. The opening and closing of the channels are triggered by changing ion concentration, and hence charge gradient, between ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |