|
Seleninic Acid
A seleninic acid is an organoselenium compound and an oxoacid with the general formula , where R ≠ H. Its structure is . It is a member of the family of organoselenium oxoacids, which also includes selenenic acids and selenonic acids, which are and , respectively. The parent member of this family of compounds is methaneseleninic acid (), also known as methylseleninic acid or "MSA". Reactions and applications in synthesis Seleninic acids (particularly areneseleninic acids) are useful catalysts for hydrogen peroxide epoxidations, Baeyer–Villiger oxidations, oxidations of thioether In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A su ...s, etc.; peroxyseleninic acids () are thought to be the active oxidants. Structure, bonding, properties Methaneseleninic acid has been characterized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methaneseleninic Acid
Methaneseleninic acid (methylseleninic acid or MSA) is an organoselenium compound, a seleninic acid with the chemical formula . Its structure is . Preparation Methaneseleninic acid is conveniently synthesized through oxidation of commercially available dimethyl diselenide by 3% hydrogen peroxide. : Seleninic acids can be prepared by oxidation of selenoesters with one equivalent of dimethyldioxirane (DMDO). Use of excess DMDO affords little studied selenonic acids (). : : Selenenic acids, formed during the ''syn''-elimination of selenoxides, undergo spontaneous disproportionation into the corresponding seleninic acids and diselenides: : Structure, bonding, properties Methaneseleninic acid, from decomposition of ''Se''-methylselenocysteine ''Se''-oxide but also available commercially, has been characterized by X-ray crystallography. The configuration about the selenium atom is pyramidal, with Se-C = 1.925(8) Å, Se-O = 1.672(7) Å, Se-OH = 1.756(7) Å, the angle OSeO = 103.0(3) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organoselenium Compound
Organoselenium compounds (or seleno-organic) are chemical compounds containing carbon-to-selenium chemical bonds. Organoselenium chemistry is the corresponding science exploring their properties and reactivity. Selenium belongs with oxygen and sulfur to the group 16 elements or chalcogens, and similarities in chemistry are to be expected. Organoselenium compounds are found at trace levels in ambient waters, soils and sediments. Selenium can exist with oxidation state −2, +2, +4, +6. Se(II) is the dominant form in organoselenium chemistry. Down the group 16 column, the bond strength becomes increasingly weaker (234 kJ/ mol for the C−Se bond and 272 kJ/mol for the C−S bond) and the bond lengths longer (C−Se 198 pm, C−S 181 pm and C−O 141 pm). Selenium compounds are more nucleophilic than the corresponding sulfur compounds and also more acidic. The p''K''a values of XH2 are 16 for oxygen, 7 for sulfur and 3.8 for selenium. In contrast to sulfoxides, the corresponding sel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenenic Acid
A selenenic acid is an organoselenium compound and an oxoacid with the general formula RSeOH, where R ≠ H. It is the first member of the family of organoselenium oxoacids, which also include seleninic acids and selenonic acids, which are RSeO2H and RSeO3H, respectively. Selenenic acids derived from selenoenzymes are thought to be responsible for the antioxidant activity of these enzymes. This functional group is sometimes called SeO-selenoperoxol. Properties In contrast to selenonic and seleninic acids, selenenic acids are unstable with respect to a self-condensation reaction to form the corresponding selenoseleninates or disproportionation into corresponding seleninic acids and diselenides: :2 RSeOH → RSe(O)SeR + H2O :2 RSeOH → RSeO2H + 1/2 RSeSeR Even the very bulky 2,4,6-tri-''tert''-butylbenzeneselenenic acid disproportionates readily. A stable selenenic acid was synthesized by burying the SeOH functional group within the cavity of a ''p''-''tert''-butyl alix[6r ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Selenonic Acid
A selenonic acid is an organoselenium compound containing the SeO3H functional group. Selenonic acids are the selenium analogs of sulfonic acids. Examples of the acid are rare. Benzeneselenonic acid is a white solid. It can be prepared by the oxidation of benzeneselenol. See also * Selenenic acid * Seleninic acid A seleninic acid is an organoselenium compound and an oxoacid with the general formula , where R ≠ H. Its structure is . It is a member of the family of organoselenium oxoacids, which also includes selenenic acids and selenonic acids, which are ... References Functional groups {{orgchem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methaneseleninic Acid
Methaneseleninic acid (methylseleninic acid or MSA) is an organoselenium compound, a seleninic acid with the chemical formula . Its structure is . Preparation Methaneseleninic acid is conveniently synthesized through oxidation of commercially available dimethyl diselenide by 3% hydrogen peroxide. : Seleninic acids can be prepared by oxidation of selenoesters with one equivalent of dimethyldioxirane (DMDO). Use of excess DMDO affords little studied selenonic acids (). : : Selenenic acids, formed during the ''syn''-elimination of selenoxides, undergo spontaneous disproportionation into the corresponding seleninic acids and diselenides: : Structure, bonding, properties Methaneseleninic acid, from decomposition of ''Se''-methylselenocysteine ''Se''-oxide but also available commercially, has been characterized by X-ray crystallography. The configuration about the selenium atom is pyramidal, with Se-C = 1.925(8) Å, Se-O = 1.672(7) Å, Se-OH = 1.756(7) Å, the angle OSeO = 103.0(3) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epoxidation
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale for many applications. In general, low molecular weight epoxides are colourless and nonpolar, and often volatile. Nomenclature A compound containing the epoxide functional group can be called an epoxy, epoxide, oxirane, and ethoxyline. Simple epoxides are often referred to as oxides. Thus, the epoxide of ethylene (C2H4) is ethylene oxide (C2H4O). Many compounds have trivial names; for instance, ethylene oxide is called "oxirane". Some names emphasize the presence of the epoxide functional group, as in the compound ''1,2-epoxyheptane'', which can also be called ''1,2-heptene oxide''. A polymer formed from epoxide precursors is called an '' epoxy'', but such materials do not contain epoxide groups (or contain only a few residual epoxy gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
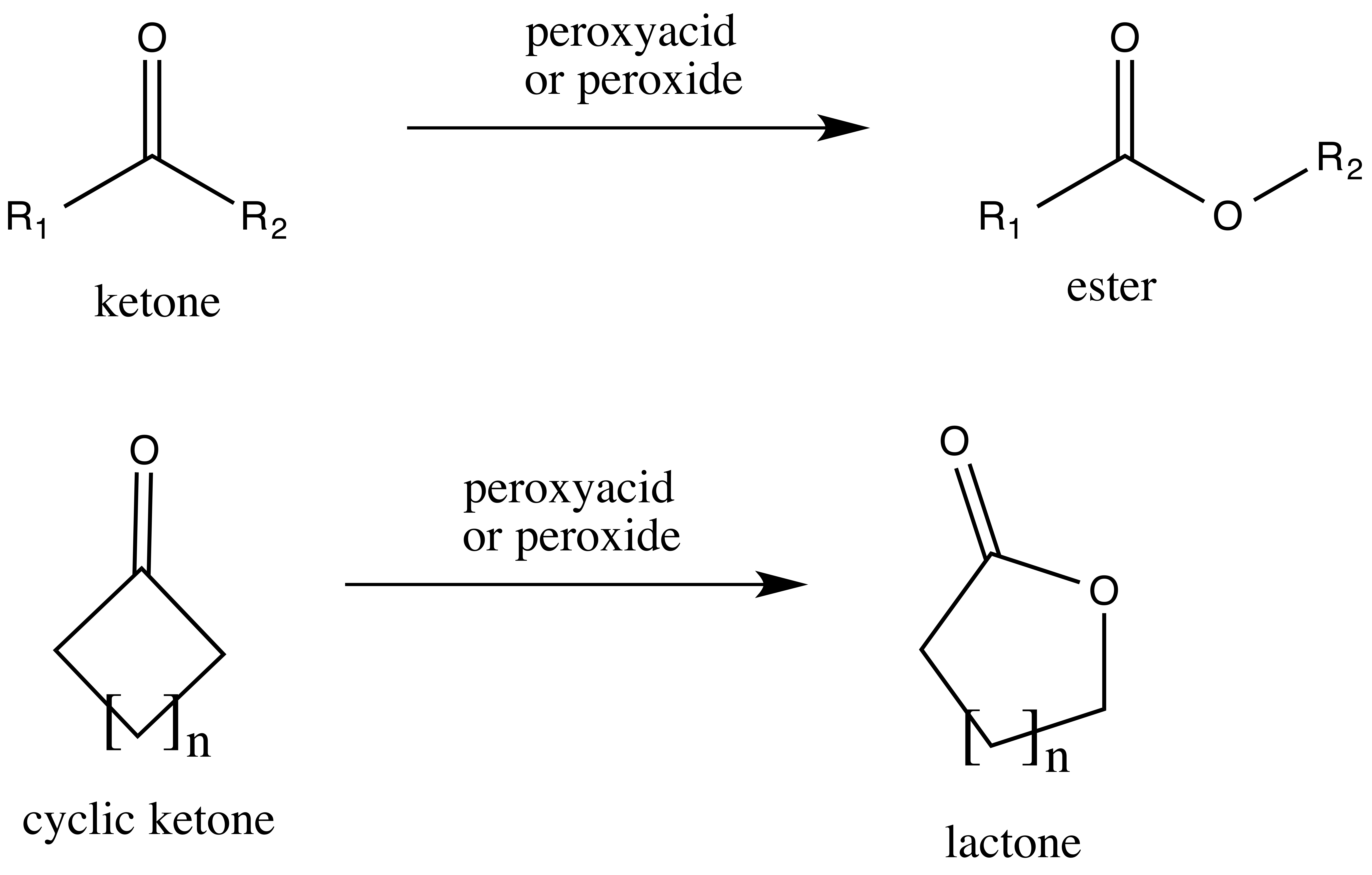
Baeyer–Villiger Oxidation
The Baeyer–Villiger oxidation is an organic reaction that forms an ester from a ketone or a lactone from a cyclic ketone, using peroxyacids or peroxides as the oxidant. The reaction is named after Adolf von Baeyer and Victor Villiger who first reported the reaction in 1899. Reaction mechanism In the first step of the reaction mechanism, the peroxyacid protonates the oxygen of the carbonyl group. This makes the carbonyl group more susceptible to be attacked by the peroxyacid. Next, the peroxyacid attacks the carbon of the carbonyl group forming what is known as the Criegee intermediate. Through a concerted mechanism, one of the substituents on the ketone group migrates to the oxygen of the peroxide group while a carboxylic acid leaves. This migration step is thought to be the rate determining step. Finally, deprotonation of the oxocarbenium ion produces the ester. The products of the Baeyer–Villiger oxidation are believed to be controlled through both primary and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioether
In organic chemistry, an organic sulfide (British English sulphide) or thioether is an organosulfur functional group with the connectivity as shown on right. Like many other sulfur-containing compounds, volatile sulfides have foul odors. A sulfide is similar to an ether except that it contains a sulfur atom in place of the oxygen. The grouping of oxygen and sulfur in the periodic table suggests that the chemical properties of ethers and sulfides are somewhat similar, though the extent to which this is true in practice varies depending on the application. Nomenclature Sulfides are sometimes called thioethers, especially in the old literature. The two organic substituents are indicated by the prefixes. (CH3)2S is called dimethylsulfide. Some sulfides are named by modifying the common name for the corresponding ether. For example, C6H5SCH3 is methyl phenyl sulfide, but is more commonly called thioanisole, since its structure is related to that for anisole, C6H5OCH3. The modern sy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Roger A
Roger is a given name, usually masculine, and a surname. The given name is derived from the Old French personal names ' and '. These names are of Germanic origin, derived from the elements ', ''χrōþi'' ("fame", "renown", "honour") and ', ' ("spear", "lance") (Hrōþigēraz). The name was introduced into England by the Normans. In Normandy, the Frankish name had been reinforced by the Old Norse cognate '. The name introduced into England replaced the Old English cognate '. ''Roger'' became a very common given name during the Middle Ages. A variant form of the given name ''Roger'' that is closer to the name's origin is ''Rodger''. Slang and other uses Roger is also a short version of the term "Jolly Roger", which refers to a black flag with a white skull and crossbones, formerly used by sea pirates since as early as 1723. From up to , Roger was slang for the word "penis". In ''Under Milk Wood'', Dylan Thomas writes "jolly, rodgered" suggesting both the sexual double entend ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen, which may be replaced by some other element or compound to serve as a functional group. Phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with C6H5− and is represented by the symbol Ph or, archaically, Φ. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. For example, triphenylmethane (Ph3 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |