|
Selected Ion Flow Tube Mass Spectrometry
Selected-ion flow-tube mass spectrometry (SIFT-MS) is a quantitative mass spectrometry technique for trace gas analysis which involves the chemical ionization Chemical ionization (CI) is a soft ionization technique used in mass spectrometry. This was first introduced by Burnaby Munson and Frank H. Field in 1966. This technique is a branch of gaseous ion-molecule chemistry. Reagent gas molecules (often ... of trace volatile compounds by selected positive precursor ions during a well-defined time period along a flow tube. Absolute concentrations of trace compounds present in air, breath or the headspace of bottled liquid samples can be calculated in real time from the ratio of the precursor and product ion signal ratios, without the need for Sample preparation (analytical chemistry), sample preparation or calibration with standard mixtures. The detection limit of commercially available SIFT-MS instruments extends to the single digit Parts-per notation, pptv range. The instrument ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SIFT-MS
Selected-ion flow-tube mass spectrometry (SIFT-MS) is a quantitative mass spectrometry technique for trace gas analysis which involves the chemical ionization of trace volatile compounds by selected positive precursor ions during a well-defined time period along a flow tube. Absolute concentrations of trace compounds present in air, breath or the headspace of bottled liquid samples can be calculated in real time from the ratio of the precursor and product ion signal ratios, without the need for sample preparation or calibration with standard mixtures. The detection limit of commercially available SIFT-MS instruments extends to the single digit pptv range. The instrument is an extension of the selected ion flow tube, SIFT, technique, which was first described in 1976 by Adams and Smith. It is a fast flow tube/ion swarm method to react positive or negative ions with atoms and molecules under truly thermalised conditions over a wide range of temperatures. It has been used extensivel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polarizability
Polarizability usually refers to the tendency of matter, when subjected to an electric field, to acquire an electric dipole moment in proportion to that applied field. It is a property of all matter, considering that matter is made up of elementary particles which have an electric charge, namely protons and electrons. When subject to an electric field, the negatively charged electrons and positively charged atomic nuclei are subject to opposite forces and undergo charge separation. Polarizability is responsible for a material's dielectric constant and, at high (optical) frequencies, its refractive index. The polarizability of an atom or molecule is defined as the ratio of its induced dipole moment to the local electric field; in a crystalline solid, one considers the dipole moment per unit cell. Note that the local electric field seen by a molecule is generally different from the macroscopic electric field that would be measured externally. This discrepancy is taken into account by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collision Theory
Collision theory is a principle of chemistry used to predict the rates of chemical reactions. It states that when suitable particles of the reactant hit each other with correct orientation, only a certain amount of collisions result in a perceptible or notable change; these successful changes are called successful collisions. The successful collisions must have enough energy, also known as activation energy, at the moment of impact to break the pre-existing bonds and form all new bonds. This results in the products of the reaction. Increasing the concentration of the reactant brings about more collisions and hence more successful collisions. Increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of collisions that have enough energy. Collision theory was proposed independently by Max Trautz in 1916 and William Lewis in 1918. When a catalyst is involved in the collision between the reactant molecules, less energy is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exothermic Reactions
In thermodynamics, an exothermic process () is a thermodynamic process or Chemical reaction, reaction that releases energy from the system to its Environment (systems), surroundings, usually in the form of heat, but also in a form of light (e.g. a spark, flame, or flash), electricity (e.g. a battery), or sound (e.g. explosion heard when burning hydrogen). The term ''exothermic'' was first coined by 19th-century French chemist Marcellin Berthelot. The opposite of an exothermic process is an endothermic process, one that absorbs energy usually in the form of heat. The concept is frequently applied in the Outline of physical science, physical sciences to chemical reactions where chemical bond energy is converted to thermal energy (heat). Two types of chemical reactions Exothermic and endothermic describe two types of chemical reactions or systems found in nature, as follows: Exothermic After an exothermic reaction, more energy has been released to the surroundings than was absor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Rate Constant
In chemical kinetics a reaction rate constant or reaction rate coefficient, ''k'', quantifies the rate and direction of a chemical reaction. For a reaction between reactants A and B to form product C the reaction rate is often found to have the form: r = k(T) mathrmm mathrm Here ''k''(''T'') is the reaction rate constant that depends on temperature, and and are the molar concentrations of substances A and B in moles per unit volume of solution, assuming the reaction is taking place throughout the volume of the solution. (For a reaction taking place at a boundary, one would use moles of A or B per unit area instead.) The exponents ''m'' and ''n'' are called partial orders of reaction and are ''not'' generally equal to the stoichiometric coefficients ''a'' and ''b''. Instead they depend on the reaction mechanism and can be determined experimentally. Elementary steps For an elementary step, there ''is'' a relationship between stoichiometry and rate law, as determined by th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mass-to-charge Ratios
The mass-to-charge ratio (''m''/''Q'') is a physical quantity relating the ''mass'' (quantity of matter) and the ''electric charge'' of a given particle, expressed in units of kilograms per coulomb (kg/C). It is most widely used in the electrodynamics of charged particles, e.g. in electron optics and ion optics. It appears in the scientific fields of electron microscopy, cathode ray tubes, accelerator physics, nuclear physics, Auger electron spectroscopy, cosmology and mass spectrometry. The importance of the mass-to-charge ratio, according to classical electrodynamics, is that two particles with the same mass-to-charge ratio move in the same path in a vacuum, when subjected to the same electric and magnetic fields. On rare occasions, the thomson has been used as its unit in the field of mass spectrometry. Some disciplines use the charge-to-mass ratio (''Q''/''m'') instead, which is the multiplicative inverse of the mass-to-charge ratio. The CODATA recommended value for an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
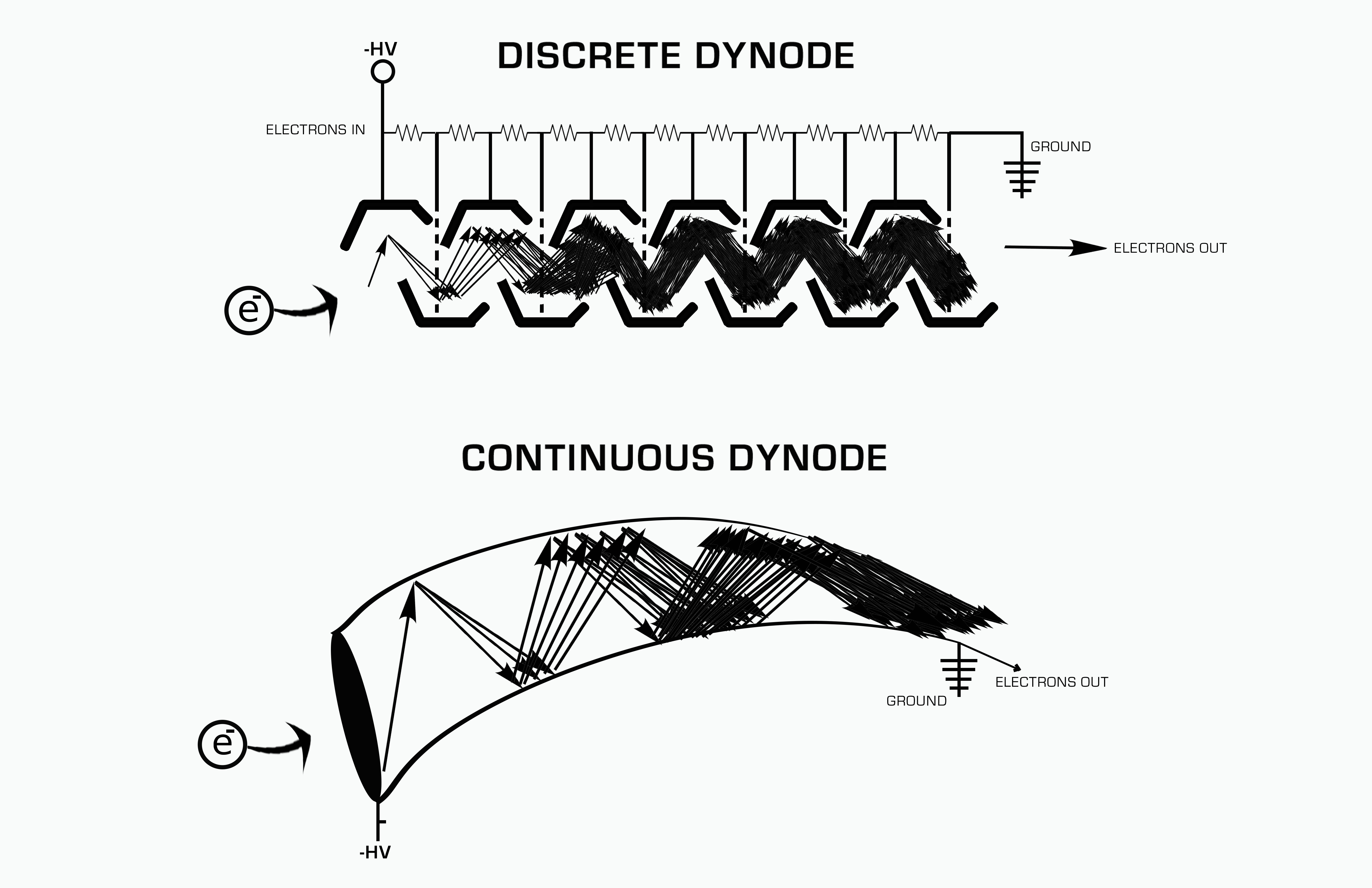
Electron Multiplier
An electron multiplier is a vacuum-tube structure that multiplies incident charges. In a process called secondary emission, a single electron can, when bombarded on secondary-emissive material, induce emission of roughly 1 to 3 electrons. If an electric potential is applied between this metal plate and yet another, the emitted electrons will accelerate to the next metal plate and induce secondary emission of still more electrons. This can be repeated a number of times, resulting in a large shower of electrons all collected by a metal anode, all having been triggered by just one. History In 1930, Russian physicist Leonid Aleksandrovitch Kubetsky proposed a device which used photocathodes combined with dynodes, or secondary electron emitters, in a single tube to remove secondary electrons by increasing the electric potential through the device. The electron multiplier can use any number of dynodes in total, which use a coefficient, σ, and created a gain of σn where n is the number ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ionization Energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged atom or molecule is called an ion. Ionization can result from the loss of an electron after collisions with subatomic particles, collisions with other atoms, molecules and ions, or through the interaction with electromagnetic radiation. Heterolytic bond cleavage and heterolytic substitution reactions can result in the formation of ion pairs. Ionization can occur through radioactive decay by the internal conversion process, in which an excited nucleus transfers its energy to one of the inner-shell electrons causing it to be ejected. Uses Everyday examples of gas ionization are such as within a fluorescent lamp or other electrical discharge lamps. It is also used in radiation detectors such as the Geiger-Müller counter or the ionization chamber. The ionizati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Proton Affinity
The proton affinity (PA, ''E''pa) of an anion or of a neutral atom or molecule is the negative of the enthalpy change in the reaction between the chemical species concerned and a proton in the gas phase: ::: A- + H+ -> HA ::: B + H+ -> BH+ These reactions are always exothermic in the gas phase, i.e. energy is released (enthalpy is negative) when the reaction advances in the direction shown above, while the proton affinity is positive. This is the same sign convention used for electron affinity. The property related to the proton affinity is the gas-phase basicity, which is the negative of the Gibbs energy for above reactions, i.e. the gas-phase basicity includes entropic terms in contrast to the proton affinity. Acid/base chemistry The higher the proton affinity, the stronger the base and the weaker the conjugate acid ''in the gas phase''. The (reportedly) strongest known base is the ortho-diethynylbenzene dianion (''E''pa = 1843 kJ/mol), followed by the methanide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Convection
Convection is single or multiphase fluid flow that occurs spontaneously due to the combined effects of material property heterogeneity and body forces on a fluid, most commonly density and gravity (see buoyancy). When the cause of the convection is unspecified, convection due to the effects of thermal expansion and buoyancy can be assumed. Convection may also take place in soft solids or mixtures where particles can flow. Convective flow may be transient (such as when a multiphase mixture of oil and water separates) or steady state (see Convection cell). The convection may be due to gravitational, electromagnetic or fictitious body forces. Heat transfer by natural convection plays a role in the structure of Earth's atmosphere, its oceans, and its mantle. Discrete convective cells in the atmosphere can be identified by clouds, with stronger convection resulting in thunderstorms. Natural convection also plays a role in stellar physics. Convection is often categorised or d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Venturi Effect
The Venturi effect is the reduction in fluid pressure that results when a fluid flows through a constricted section (or choke) of a pipe. The Venturi effect is named after its discoverer, the 18th century Italian physicist, Giovanni Battista Venturi. Background In inviscid fluid dynamics, an incompressible fluid's velocity must ''increase'' as it passes through a constriction in accord with the principle of mass continuity, while its static pressure must ''decrease'' in accord with the principle of conservation of mechanical energy (Bernoulli's principle). Thus, any gain in kinetic energy a fluid may attain by its increased velocity through a constriction is balanced by a drop in pressure. By measuring pressure, the flow rate can be determined, as in various flow measurement devices such as Venturi meters, Venturi nozzles and orifice plates. Referring to the adjacent diagram, using Bernoulli's equation in the special case of steady, incompressible, inviscid flows (such as t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |