|
Salvia Nemorosa
''Salvia nemorosa'', the woodland sage, Balkan clary, blue sage or wild sage, is a hardy herbaceous perennial plant native to a wide area of central Europe and Western Asia. It is an attractive plant that is easy to grow and propagate, with the result that it has been passed around by gardeners for many years. Its wide distribution, long history, and the ease with which it hybridizes have resulted in many cultivars and hybrids—along with problems in clearly identifying the hybrids and their relationship with ''S. nemorosa''. It was named and described by Carl Linnaeus in 1762, with ''nemorosa'' ("of woods") referring to its typical habitat in groves and woods. In northern Britain, ''Salvia nemorosa'' and ''Salvia pratensis'' are both in danger of disappearing due to depredation from slugs. Description The many inflorescences have closely spaced whorls of small flowers with brightly colored calyces. Cultivation There are numerous cultivars widely grown in horticulture. Many o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carl Linnaeus
Carl Linnaeus (; 23 May 1707 – 10 January 1778), also known after his ennoblement in 1761 as Carl von Linné Blunt (2004), p. 171. (), was a Swedish botanist, zoologist, taxonomist, and physician who formalised binomial nomenclature, the modern system of naming organisms. He is known as the "father of modern taxonomy". Many of his writings were in Latin; his name is rendered in Latin as and, after his 1761 ennoblement, as . Linnaeus was born in Råshult, the countryside of Småland, in southern Sweden. He received most of his higher education at Uppsala University and began giving lectures in botany there in 1730. He lived abroad between 1735 and 1738, where he studied and also published the first edition of his ' in the Netherlands. He then returned to Sweden where he became professor of medicine and botany at Uppsala. In the 1740s, he was sent on several journeys through Sweden to find and classify plants and animals. In the 1750s and 1760s, he continued to collect an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flavonoids
Flavonoids (or bioflavonoids; from the Latin word ''flavus'', meaning yellow, their color in nature) are a class of polyphenolic secondary metabolites found in plants, and thus commonly consumed in the diets of humans. Chemically, flavonoids have the general structure of a 15-carbon skeleton, which consists of two phenyl rings (A and B) and a heterocyclic ring (C, the ring containing the embedded oxygen). This carbon structure can be abbreviated C6-C3-C6. According to the IUPAC nomenclature, they can be classified into: *flavonoids or bioflavonoids *isoflavonoids, derived from 3-phenyl chromen-4-one (3-phenyl-1,4-benzopyrone) structure *neoflavonoids, derived from 4-phenylcoumarine (4-phenyl-1,2-benzopyrone) structure The three flavonoid classes above are all ketone-containing compounds and as such, anthoxanthins ( flavones and flavonols). This class was the first to be termed bioflavonoids. The terms flavonoid and bioflavonoid have also been more loosely used to describe non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
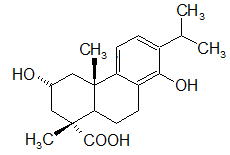
β-sitosterol
β-sitosterol (beta-sitosterol) is one of several phytosterols (plant sterols) with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols. Natural occurrences and food β-sitosterol is widely distributed in the plant kingdom. It is found in vegetable oil, nuts, avocados, and derived prepared foods such as salad dressings. Human research β-sitosterol is being studied for its potential to reduce benign prostatic hyperplasia (BPH) and blood cholesterol levels. Genetic disorder While plant sterols are usually beneficial, there is a rare autosomal recessive genetic disorder phytosterolemia which causes over-absorption of phytosterols. Precursor of anabolic steroid boldenone Being a steroid, β-sitosterol is a precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oleanolic Acid
Oleanolic acid or oleanic acid is a naturally occurring pentacyclic triterpenoid related to betulinic acid. It is widely distributed in food and plants where it exists as a free acid or as an aglycone of triterpenoid saponins. Natural occurrence Oleanolic acid can be found in olive oil, ''Phytolacca americana'' (American pokeweed), and ''Syzygium'' spp, garlic, etc. It was first studied and isolated from several plants, including ''Olea europaea'' (leaves, fruit), ''Rosa woodsii'' (leaves), ''Prosopis glandulosa'' (leaves and twigs), '' Phoradendron juniperinum'' (whole plant), '' Syzygium claviflorum'' (leaves), '' Hyptis capitata'' (whole plant), ''Mirabilis jalapa'') and ''Ternstroemia gymnanthera'' (aerial part). Other ''Syzygium'' species including java apple (''Syzygium samarangense'') and rose apples contain it, as does ''Ocimum tenuiflorum'' (holy basil). Biosynthesis of oleanolic acids Oleanolic acid biosynthesis starts with mevalonate to create squalene. Squalen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ursolic Acid
Ursolic acid (sometimes referred to as urson, prunol, malol, or 3β-hydroxyurs-12-en-28-oic acid), is a pentacyclic triterpenoid identified in the epicuticular waxes of apples as early as 1920 and widely found in the peels of fruits, as well as in herbs and spices like rosemary and thyme. Natural occurrence Ursolic acid is present in many plants, such as ''Mirabilis jalapa'', as well as in many fruits and herbs used in daily life (e.g. apples, basil and holy basil, bilberries, cranberries, elder flower, peppermint, rosemary, lavender, oregano, thyme, hawthorn, and prunes). Apple peels contain large quantities of ursolic acid and related compounds. Potential biochemical effects A number of potential biochemical effects of ursolic acid have been investigated, but there has been no clinical study demonstrating benefits to human health. ''In vitro'', ursolic acid inhibits the proliferation of various cancer cell types by inhibiting the STAT3 activation pathway, and may also d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
α-amyrin
The amyrins are three closely related natural chemical compounds of the triterpene class. They are designated α-amyrin (ursane skeleton), β-amyrin (oleanane skeleton) and δ-amyrin. Each is a pentacyclic triterpenol with the chemical formula C30H50O. They are widely distributed in nature and have been isolated from a variety of plant sources such as epicuticular wax. In plant biosynthesis, α-amyrin is the precursor of ursolic acid and β-amyrin is the precursor of oleanolic acid. All three amyrins occur in the surface wax of tomato fruit. α-Amyrin is found in dandelion coffee. A study demonstrated that α,β-amyrin exhibits long-lasting antinociceptive and anti-inflammatory properties in 2 models of persistent nociception via activation of the cannabinoid receptors CB1 and CB2 and by inhibiting the production of cytokines and expression of NF-κB, CREB and cyclooxygenase 2 Prostaglandin-endoperoxide synthase 2 (prostaglandin G/H synthase and cyclooxygenase) (The HUGO ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosides
In chemistry, a glycoside is a molecule in which a sugar is bound to another functional group via a glycosidic bond. Glycosides play numerous important roles in living organisms. Many plants store chemicals in the form of inactive glycosides. These can be activated by enzyme hydrolysis, which causes the sugar part to be broken off, making the chemical available for use. Many such plant glycosides are used as medications. Several species of ''Heliconius'' butterfly are capable of incorporating these plant compounds as a form of chemical defense against predators. In animals and humans, poisons are often bound to sugar molecules as part of their elimination from the body. In formal terms, a glycoside is any molecule in which a sugar group is bonded through its anomeric carbon to another group via a glycosidic bond. Glycosides can be linked by an O- (an ''O-glycoside''), N- (a ''glycosylamine''), S-(a ''thioglycoside''), or C- (a '' C-glycoside'') glycosidic bond. According to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |