|
Saegusa–Ito Oxidation
The Saegusa–Ito oxidation is a chemical reaction used in organic chemistry. It was discovered in 1978 by Takeo Saegusa and Yoshihiko Ito as a method to introduce α-β unsaturation in carbonyl compounds. The reaction as originally reported involved formation of a silyl enol ether followed by treatment with palladium(II) acetate and benzoquinone to yield the corresponding enone. The original publication noted its utility for regeneration of unsaturation following 1,4-addition with nucleophiles such as organocuprates. For acyclic substrates the reaction yields the thermodynamic E-olefin product exclusively. This discovery was preceded nearly eight years earlier by a report that treatment of unactivated ketones with palladium acetate yielded the same products in low yields. The major improvement provided by Saegusa and Ito was the recognition that the enol form was the reactive species, developing a method based on silyl enol ethers. Benzoquinone is actually not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Reaction
A chemical reaction is a process that leads to the IUPAC nomenclature for organic transformations, chemical transformation of one set of chemical substances to another. Classically, chemical reactions encompass changes that only involve the positions of electrons in the forming and breaking of chemical bonds between atoms, with no change to the Atomic nucleus, nuclei (no change to the elements present), and can often be described by a chemical equation. Nuclear chemistry is a sub-discipline of chemistry that involves the chemical reactions of unstable and radioactive Chemical element, elements where both electronic and nuclear changes can occur. The substance (or substances) initially involved in a chemical reaction are called reagent, reactants or reagents. Chemical reactions are usually characterized by a chemical change, and they yield one or more Product (chemistry), products, which usually have properties different from the reactants. Reactions often consist of a sequence o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
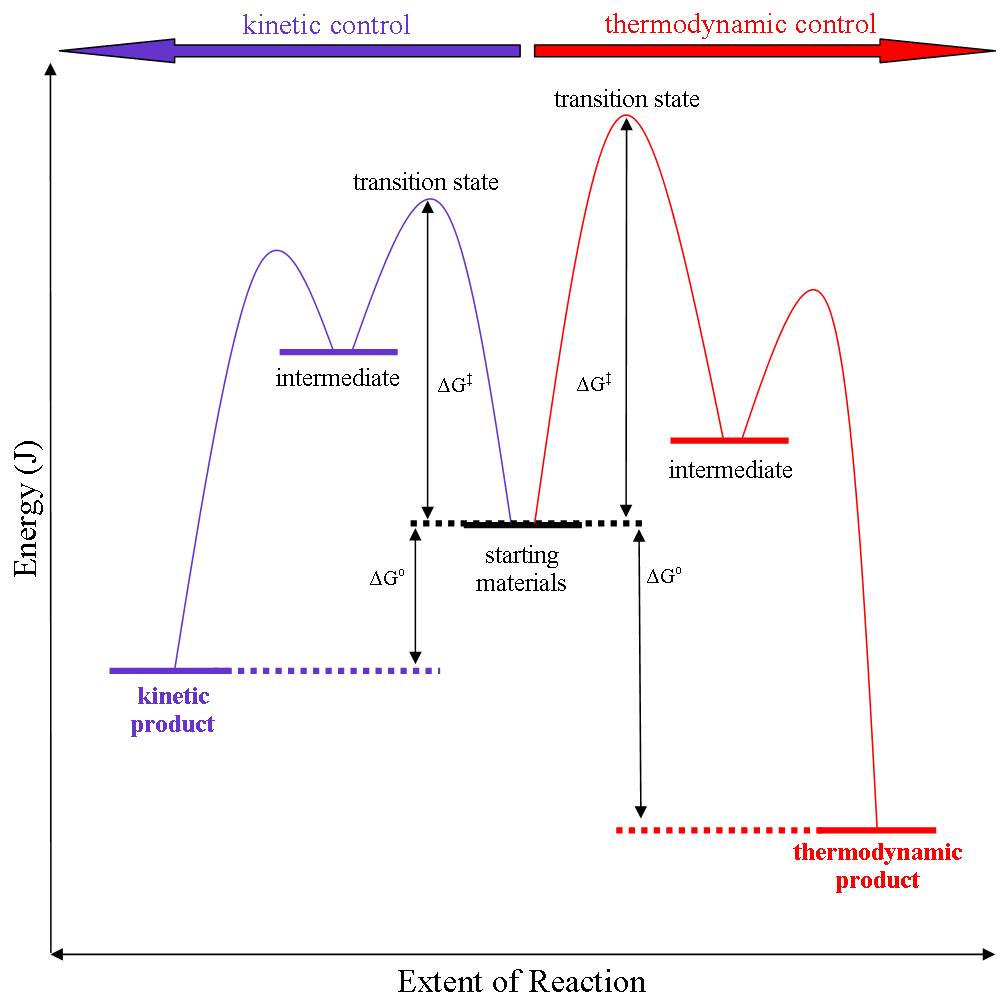
Thermodynamic Versus Kinetic Reaction Control
Thermodynamic reaction control or kinetic reaction control in a chemical reaction can decide the composition in a reaction product mixture when competing pathways lead to different products and the reaction conditions influence the conversion (chemistry), selectivity or stereoselectivity. The distinction is relevant when product A forms faster than product B because the activation energy for product A is lower than that for product B, yet product B is more stable. In such a case A is the kinetic product and is favoured under kinetic control and B is the thermodynamic product and is favoured under thermodynamic control.Introduction to Organic Chemistry I, Seth Robert Elsheimer, Blackwell Publishing, 2000 The conditions of the reaction, such as temperature, pressure, or solvent, affect which reaction pathway may be favored: either the kinetically controlled or the thermodynamically controlled one. Note this is only true if the activation energy of the two pathways differ, with one p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Equilibrium
In a chemical reaction, chemical equilibrium is the state in which both the reactants and products are present in concentrations which have no further tendency to change with time, so that there is no observable change in the properties of the system. This state results when the forward reaction proceeds at the same rate as the reverse reaction. The reaction rates of the forward and backward reactions are generally not zero, but they are equal. Thus, there are no net changes in the concentrations of the reactants and products. Such a state is known as dynamic equilibrium. Historical introduction The concept of chemical equilibrium was developed in 1803, after Berthollet found that some chemical reactions are reversible. For any reaction mixture to exist at equilibrium, the rates of the forward and backward (reverse) reactions must be equal. In the following chemical equation, arrows point both ways to indicate equilibrium. A and B are reactant chemical species, S and T are p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water and other trace elements. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is fundamental to all forms of life. When bound to coenzyme A, it is central to the metabolism of carbohydrates and fats. The global ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reductive Elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and is often the product-forming step in many catalytic processes. Since oxidative addition and reductive elimination are reverse reactions, the same mechanisms apply for both processes, and the product equilibrium depends on the thermodynamics of both directions. General information Reductive elimination is often seen in higher oxidation states, and can involve a two-electron change at a single metal center (mononuclear) or a one-electron change at each of two metal centers (binuclear, dinuclear, or bimetallic). For mononuclear reductive elimination, the oxidation state of the metal decreases by two, while the d-electron count of the metal increases by two. This pathway is common for d8 metals Ni(II), Pd(II), and Au(III) and d6 metals Pt(I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
β-hydride Elimination
β-Hydride elimination is a reaction in which an alkyl group bonded to a metal centre is converted into the corresponding metal-bonded hydride and an alkene. The alkyl must have hydrogens on the β-carbon. For instance butyl groups can undergo this reaction but methyl groups cannot. The metal complex must have an empty (or vacant) site ''cis'' to the alkyl group for this reaction to occur. Moreover, for facile cleavage of the C–H bond, a d electron pair is needed for donation into the σ* orbital of the C–H bond. Thus, d0 metals alkyls are generally more stable to β-hydride elimination than d2 and higher metal alkyls and may form isolable agostic complexes, even if an empty coordination site is available. The β-hydride elimination can either be a vital step in a reaction or an unproductive side reaction. The Shell higher olefin process relies on β-hydride elimination to produce α-olefins which are used to produce detergents. Illustrative of a sometimes undesirable � ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reaction Mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical change occurs. A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage of an overall chemical reaction. The detailed steps of a reaction are not observable in most cases. The conjectured mechanism is chosen because it is thermodynamically feasible, and has experimental support in isolated intermediates (see next section) or other quantitative and qualitative characteristics of the reaction. It also describes each reactive intermediate, activated complex, and transition state, and which bonds are broken (and in what order), and which bonds are formed (and in what order). A complete mechanism must also explain the reason for the reactants and catalyst used, the stereochemistry observed in reactants and products, all products formed and the amount of each. The electron or arrow pushing method is often used in i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalytic
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usually gaseous or liquid) as the reactant, or heterogeneous, whose components are not in the same phase. Enzymes and other biocatalysts are often considered as a third category. Catalysis is ubiquitous in chemical industry of all kinds. Estimates are that 90% of all commercially produced chemical products involve catalysts at some stag ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stoichiometry
Stoichiometry refers to the relationship between the quantities of reactants and products before, during, and following chemical reactions. Stoichiometry is founded on the law of conservation of mass where the total mass of the reactants equals the total mass of the products, leading to the insight that the relations among quantities of reactants and products typically form a ratio of positive integers. This means that if the amounts of the separate reactants are known, then the amount of the product can be calculated. Conversely, if one reactant has a known quantity and the quantity of the products can be empirically determined, then the amount of the other reactants can also be calculated. This is illustrated in the image here, where the balanced equation is: : Here, one molecule of methane reacts with two molecules of oxygen gas to yield one molecule of carbon dioxide and two molecules of water. This particular chemical equation is an example of complete combustion. St ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chem
Chem may refer to: * Chemistry practical waali mam *Chemistry *Chemical * ''Chem'' (journal), a scientific journal published by Cell Press *Post apocalyptic slang for "drugs", medicinal or otherwise in the Fallout video game series. In Ancient Egyptian usage: * ''Khem'' (also spelt ''Chem''), the Egyptian word for "black" * Min (god), in the past erroneously named ''Khem'' CHEM may refer to : *A metabolic panel: for instance, CHEM-7, which is the basic metabolic panel *CHEM-DT CHEM-DT is the TVA owned-and-operated television station in Trois-Rivières, Quebec, Canada. It broadcasts a high-definition digital signal on VHF channel 8 from a transmitter on Rue Principale in Notre-Dame-du-Mont-Carmel. Owned by the Grou ..., a Canadian television channel See also * Chemo (other) * Kemi, a place in Finland {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |