|
Sphingomyelin
Sphingomyelin (SPH, ) is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a phosphoethanolamine head group; therefore, sphingomyelins can also be classified as sphingophospholipids. In humans, SPH represents ~85% of all sphingolipids, and typically makes up 10–20 mol % of plasma membrane lipids. Sphingomyelin was first isolated by German chemist Johann L.W. Thudicum in the 1880s. The structure of sphingomyelin was first reported in 1927 as N-acyl-sphingosine-1-phosphorylcholine. Sphingomyelin content in mammals ranges from 2 to 15% in most tissues, with higher concentrations found in nerve tissues, red blood cells, and the ocular lenses. Sphingomyelin has significant structural and functional roles in the cell. It is a plasma membrane component and participates in many signaling pathways. The metabolism of sphingomyelin c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphingomyelin Core Structure Colored
Sphingomyelin (SPH, ) is a type of sphingolipid found in animal cell membranes, especially in the membranous myelin sheath that surrounds some nerve cell axons. It usually consists of phosphocholine and ceramide, or a ethanolamine, phosphoethanolamine head group; therefore, sphingomyelins can also be classified as sphingophospholipids. In humans, SPH represents ~85% of all sphingolipids, and typically makes up 10–20 mol % of plasma membrane lipids. Sphingomyelin was first isolated by Germans, German chemist Johann Ludwig Wilhelm Thudichum, Johann L.W. Thudicum in the 1880s. The structure of sphingomyelin was first reported in 1927 as N-acyl-sphingosine-1-phosphorylcholine. Sphingomyelin content in mammals ranges from 2 to 15% in most tissues, with higher concentrations found in nerve tissues, red blood cells, and the ocular lenses. Sphingomyelin has significant structural and functional roles in the cell. It is a plasma membrane component and participates in many signa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ceramide
Ceramides are a family of waxy lipid molecules. A ceramide is composed of sphingosine and a fatty acid joined by an amide bond. Ceramides are found in high concentrations within the cell membrane of Eukaryote, eukaryotic cells, since they are component lipids that make up sphingomyelin, one of the major lipids in the lipid bilayer. Contrary to previous assumptions that ceramides and other sphingolipids found in cell membrane were purely supporting structural elements, ceramide can participate in a variety of cellular lipid signaling, signaling: examples include regulating cell differentiation, differentiation, cell proliferation, proliferation, and programmed cell death (PCD) of Cell (biology), cells. The word ''ceramide'' comes from the Latin ''cera'' (wax) and ''amide''. Ceramide is a component of vernix caseosa, the waxy or cheese-like white substance found coating the skin of newborn human infants. Pathways for ceramide synthesis There are three major pathways of ceramide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphingolipids General Structures
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, which are a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sphinx because of their enigmatic nature. These compounds play important roles in signal transduction and cell recognition. Sphingolipidoses, or disorders of sphingolipid metabolism, have particular impact on neural tissue. A sphingolipid with a terminal hydroxyl group is a ceramide. Other common groups bonded to the terminal oxygen atom include phosphocholine, yielding a sphingomyelin, and various sugar monomers or dimers, yielding cerebrosides and globosides, respectively. Cerebrosides and globosides are collectively known as glycosphingolipids. Structure The long-chain bases, sometimes simply known as sphingoid bases, are the first non-transient products of '' de novo'' sphingolipid synthesis in both yeast and mammals. These co ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Myelin Sheath
Myelin Sheath ( ) is a lipid-rich material that in most vertebrates surrounds the axons of neurons to insulate them and increase the rate at which electrical impulses (called action potentials) pass along the axon. The myelinated axon can be likened to an electrical wire (the axon) with insulating material (myelin) around it. However, unlike the plastic covering on an electrical wire, myelin does not form a single long sheath over the entire length of the axon. Myelin ensheaths part of an axon known as an internodal segment, in multiple myelin layers of a tightly regulated internodal length. The ensheathed segments are separated at regular short unmyelinated intervals, called nodes of Ranvier. Each node of Ranvier is around one micrometre long. Nodes of Ranvier enable a much faster rate of conduction known as saltatory conduction where the action potential recharges at each node to jump over to the next node, and so on till it reaches the axon terminal. At the terminal the a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sphingosine
Sphingosine (2-amino-4-trans-octadecene-1,3-diol) is an 18-carbon amino alcohol with an unsaturated hydrocarbon chain, which forms a primary part of sphingolipids, a class of cell membrane lipids that include sphingomyelin, an important phospholipid. Functions Sphingosine can be phosphorylated in vivo via two kinases, sphingosine kinase type 1 and sphingosine kinase type 2. This leads to the formation of sphingosine-1-phosphate, a potent signaling lipid. Sphingolipid metabolites, such as ceramides, sphingosine and sphingosine-1-phosphate, are lipid signaling molecules involved in diverse cellular processes. Biosynthesis Sphingosine is synthesized from palmitoyl CoA and serine in a condensation required to yield dihydrosphingosine. Dehydrosphingosine is then reduced by NADPH to dihydrosphingosine (sphinganine), acylated to dihydroceramide and finally oxidized by FAD to ceramide. Sphingosine is then solely formed via degradation of sphingolipid in the lysosome. Gal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphatidylcholine
Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can easily be obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoylphosphatidylcholine (lecithin) is a major component of the pulmonary surfactant, and is often used in the lecithin–sphingomyelin ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria, including ''Escherichia coli ''Escherichia coli'' ( )Wells, J. C. (2000) Longman Pronunciation Dictionary. Harlow ngland Pearson Education Ltd. is a gram-negative, facultative anaerobic, rod-shaped, coliform bacterium of the genus '' Esch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Johann Ludwig Wilhelm Thudichum
Johann Ludwig Wilhelm Thudichum, also known as John Louis William Thudichum (August 27, 1829, Büdingen – September 7, 1901) was a German-born physician and biochemist. From 1847 he studied medicine at the University of Giessen, where he worked after hours in the laboratory of Justus von Liebig (1803–1873). In 1853 he moved to London, where he worked for the remainder of his career. Thudichum was a pioneer of British biochemistry, and a founder of "brain chemistry". He is credited with conducting chemical analyses of over one thousand human and animal brains. In his research, he isolated and characterized numerous compounds of the brain, such as cephalin, sphingomyelin, galactose, lactic acid and sphingosine. In 1884 he explained his findings in a publication titled "A Treatise on the Chemical Constitution of the Brain", a book that was widely criticized and rejected at the time by many in the scientific community. After his death, Thudichum's discoveries were realized ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-serine
Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC. Occurrence This compound is one of the proteinogenic amino acids. Only the L- stereoisomer appears naturally in proteins. It is not essential to the human diet, since it is synthesized in the body from other metabolites, including glycine. Serine was first obtained from silk protein, a particularly rich source, in 1865 by Emil Cramer. Its name is derived from the Latin for silk, '' sericum''. Serine's structure was established in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serine Palmitoyltransferase
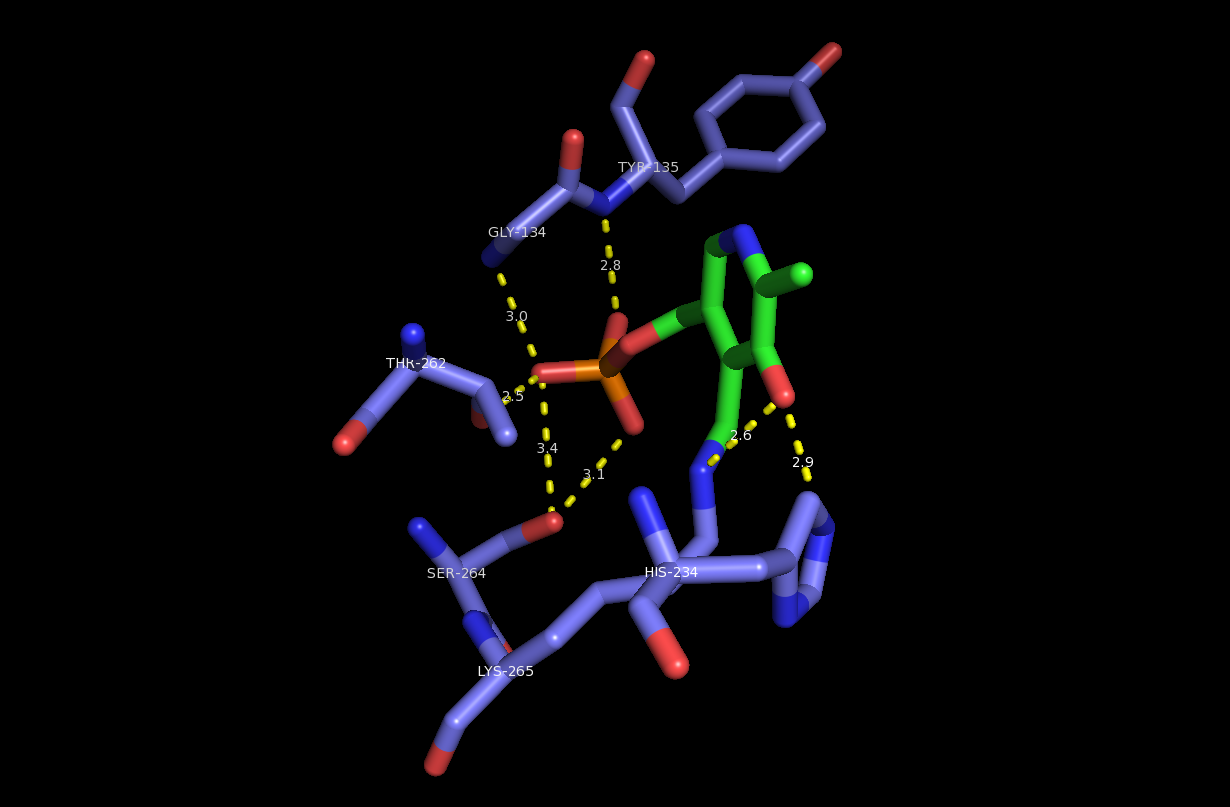
In enzymology, a serine ''C''-palmitoyltransferase () is an enzyme that catalyzes the chemical reaction: :palmitoyl-CoA + -serine \rightleftharpoons CoA + 3-dehydro--sphinganine + CO2 Thus, the two substrates of this enzyme are palmitoyl-CoA and -serine, whereas its 3 products are CoA, 3-dehydro--sphinganine, and CO2. This reaction is a key step in the biosynthesis of sphingosine which is a precursor of many other sphingolipids. This enzyme participates in sphingolipid metabolism. It employs one cofactor, pyridoxal phosphate. Nomenclature This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. The systematic name of this enzyme class is palmitoyl-CoA:L-serine ''C''-palmitoyltransferase (decarboxylating). Other names in common use include: * serine palmitoyltransferase, * SPT, 3-oxosphinganine synthetase, and * acyl-CoA:serine C-2 acyltransferase decarboxylating. Structure Serine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Palmitoyl-CoA
Palmitoyl-CoA is an acyl-CoA thioester. It is an "activated" form of palmitic acid and can be transported into the mitochondrial matrix by the carnitine shuttle system (which transports fatty acyl-CoA molecules into the mitochondria), and once inside can participate in beta-oxidation. Alternatively, palmitoyl-CoA is used as a substrate in the biosynthesis of sphingosine (this biosynthetic pathway does not require transfer into the mitochondria). Biosynthesis Palmitoyl CoA formed from palmitic acid, in the reaction below. : This reaction is often referred to as the "activation" of a fatty acid. The activation is catalyzed by palmitoyl-coenzyme A synthetase and the reaction proceeds through a two step mechanism, in which palmitoyl-AMP is an intermediate. The reaction is driven to completion by the exergonic hydrolysis of pyrophosphate. The activation of fatty acids occurs in the cytosol and beta-oxidation occurs in the mitochondria. However, long chain fatty acyl-CoA cannot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |