|
Solar Chemical
Solar chemical refers to a number of possible processes that harness solar energy by absorbing sunlight in a chemical reaction. The idea is conceptually similar to photosynthesis in plants, which converts solar energy into the chemical bonds of glucose molecules, but without using living organisms, which is why it is also called artificial photosynthesis. A promising approach is to use focused sunlight to provide the energy needed to split water into its constituent hydrogen and oxygen in the presence of a metallic catalyst such as zinc. This is normally done in a two-step process so that hydrogen and oxygen are not produced in the same chamber, which creates an explosion hazard. Another approach involves taking the hydrogen created in this process and combining it with carbon dioxide to create methane. The benefit of this approach is that there is an established infrastructure for transporting and burning methane for power generation, which is not true for hydrogen. One main drawba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Energy
Solar energy is radiant light and heat from the Sun that is harnessed using a range of technologies such as solar power to generate electricity, solar thermal energy (including solar water heating), and solar architecture. It is an essential source of renewable energy, and its technologies are broadly characterized as either passive solar or active solar depending on how they capture and distribute solar energy or convert it into solar power. Active solar techniques include the use of photovoltaic systems, concentrated solar power, and solar water heating to harness the energy. Passive solar techniques include orienting a building to the Sun, selecting materials with favorable thermal mass or light-dispersing properties, and designing spaces that naturally circulate air. The large magnitude of solar energy available makes it a highly appealing source of electricity. In 2020 solar energy has been the cheapest source of Electricity. In Saudi Arabia a power purchase agreemen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Activation Energy
In chemistry and physics, activation energy is the minimum amount of energy that must be provided for compounds to result in a chemical reaction. The activation energy (''E''a) of a reaction is measured in joules per mole (J/mol), kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius. Other uses Although less commonly used, activation energy also applies to nuclear reactions and various other physical phenomena. Te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Density
In physics, energy density is the amount of energy stored in a given system or region of space per unit volume. It is sometimes confused with energy per unit mass which is properly called specific energy or . Often only the ''useful'' or extractable energy is measured, which is to say that inaccessible energy (such as rest mass energy) is ignored. In cosmological and other general relativistic contexts, however, the energy densities considered are those that correspond to the elements of the stress–energy tensor and therefore do include mass energy as well as energy densities associated with pressure. Energy per unit volume has the same physical units as pressure and in many situations is synonymous. For example, the energy density of a magnetic field may be expressed as and behaves like a physical pressure. Likewise, the energy required to compress a gas to a certain volume may be determined by multiplying the difference between the gas pressure and the external pressure by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Nanotube
A scanning tunneling microscopy image of a single-walled carbon nanotube Rotating single-walled zigzag carbon nanotube A carbon nanotube (CNT) is a tube made of carbon with diameters typically measured in nanometers. ''Single-wall carbon nanotubes'' (''SWCNTs'') are one of the allotropes of carbon, intermediate between fullerene cages and flat graphene, with diameters in the range of a nanometre. Although not made this way, single-wall carbon nanotubes can be idealized as cutouts from a two-dimensional Hexagonal tiling, hexagonal lattice of carbon atoms rolled up along one of the Bravais lattice vectors of the hexagonal lattice to form a hollow cylinder. In this construction, periodic boundary conditions are imposed over the length of this roll-up vector to yield a helical lattice of seamlessly bonded carbon atoms on the cylinder surface. ''Multi-wall carbon nanotubes'' (''MWCNTs'') consisting of nested single-wall carbon nanotubes weakly bound together by van der Waals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Joule Per Mole
The joule per mole (symbol: J·mol−1 or J/mol) is the unit of energy per amount of substance in the International System of Units (SI), such that energy is measured in joules, and the amount of substance is measured in moles. It is also an SI derived unit of molar thermodynamic energy defined as the energy equal to one joule in one mole of substance. For example, the Gibbs free energy of a compound in the area of thermochemistry is often quantified in units of kilojoules per mole (symbol: kJ·mol−1 or kJ/mol), with 1 kilojoule = 1000 joules. Physical quantities measured in J·mol−1 usually describe quantities of energy transferred during phase transformations or chemical reactions. Division by the number of moles facilitates comparison between processes involving different quantities of material and between similar processes involving different types of materials. The precise meaning of such a quantity is dependent on the context (what substances are involved, circumst ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quadricyclane
Quadricyclane is a strained, multi-cyclic hydrocarbon with the formula CH2(CH)6. A white volatile colorless liquid, it is highly strained molecule (78.7 kcal/mol). Isomerization of quadricyclane proceeds slowly at low temperatures.Petrov, V. A; Vasil’ev, N. V. “Synthetic Chemistry of Quadricyclane.” ''Current Organic Synthesis'' 3 (2006): 215–259 Because of quadricyclane’s strained structure and thermal stability, it has been studied extensively. Preparation Quadricyclane is produced by the irradiation of norbornadiene (bicyclo .2.1epta-2,5-diene) in the presence of Michler's ketone or ethyl Michler's ketone. Other sensitizers, such as acetone, benzophenone, acetophenone, etc., may be used but with a lesser yield. The yield is higher for freshly distilled norbornadiene, but commercial reagents will suffice. : Proposed applications to solar energy The conversion of norbornadiene into quadricyclane is achievd with ~300nm UV radiation. . When converted back to norbo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
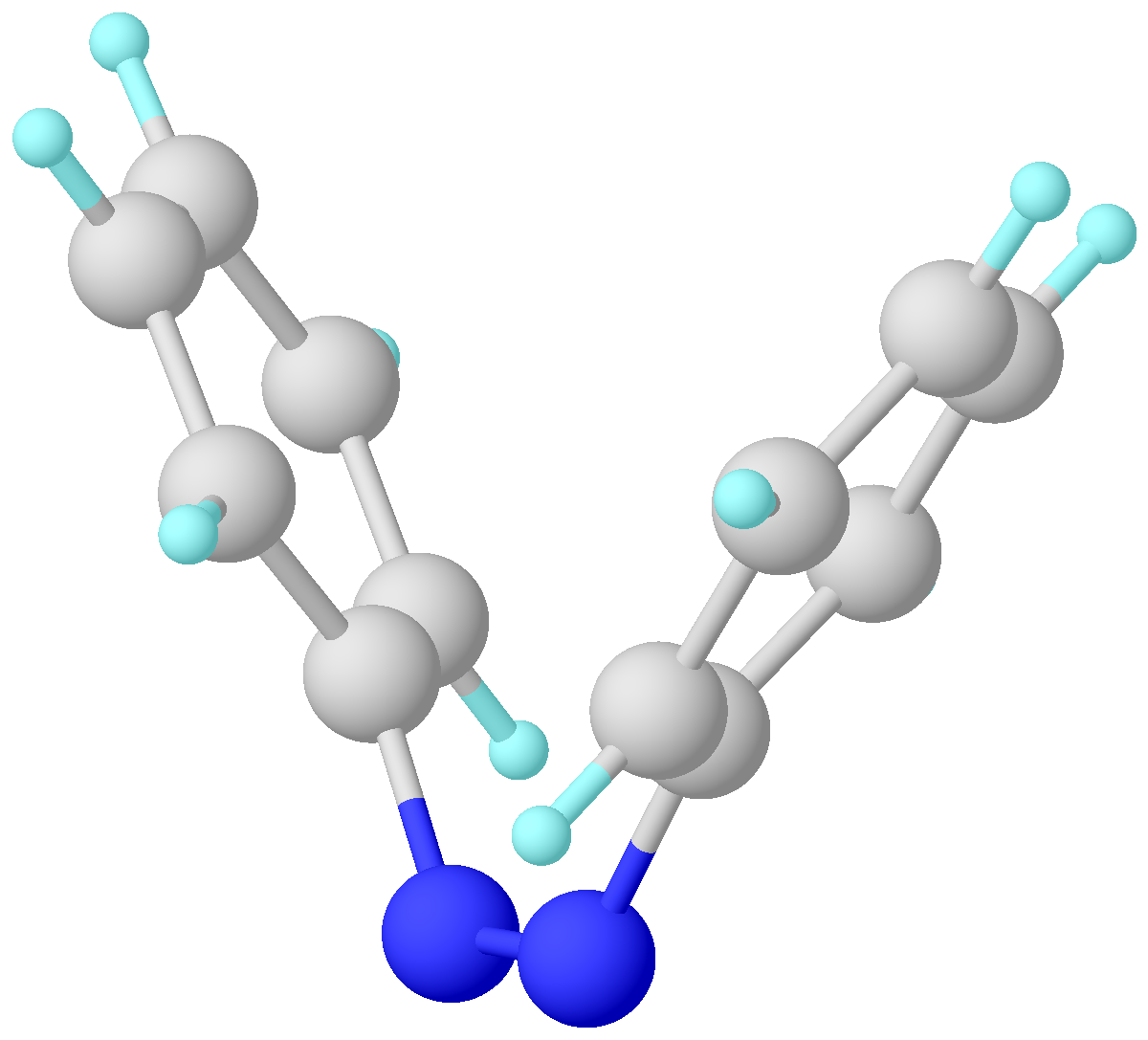
Azobenzene
Azobenzene is a photoswitchable chemical compound composed of two phenyl rings linked by a N=N double bond. It is the simplest example of an aryl azo compound. The term 'azobenzene' or simply 'azo' is often used to refer to a wide class of similar compounds. These azo compounds are considered as derivatives of diazene (diimide), and are sometimes referred to as 'diazenes'. The diazenes absorb light strongly and are common dyes. Structure and synthesis ''trans''-Azobenzene is planar. The N-N distance is 1.189 Å. ''cis''-Azobenzene is nonplanar with a C-N=N-C dihedral angle of 173.5°. The N-N distance is 1.251 Å. Azobenzene was first described by Eilhard Mitscherlich in 1834. Yellowish-red crystalline flakes of azobenzene were obtained in 1856. Its original preparation is similar to the modern one. According to the 1856 method, nitrobenzene is reduced by iron filings in the presence of acetic acid. In the modern synthesis, zinc is the reductant in the presence of a base. I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Norbornadiene
Norbornadiene is an organic compound In chemistry, organic compounds are generally any chemical compounds that contain carbon-hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The ... and a bicyclic hydrocarbon. Norbornadiene is of interest as a metal-binding ligand, whose complexes are useful for homogeneous catalysis. It has been intensively studied owing to its high reactivity and distinctive structural property of being a diene that cannot isomerization, isomerize (isomers would be anti-Bredt alkenes). Norbornadiene is also a useful Diels–Alder_reaction#The_dienophile, dienophile in Diels-Alder reactions. Synthesis Norbornadiene can be formed by a Diels-Alder reaction between cyclopentadiene and acetylene : Reactions Quadricyclane, a valence isomer, can be obtained from norbornadiene by a photochemical reaction when assisted by a photochemical sensitizer, sensiti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Fuel
A solar fuel is a synthetic chemical fuel produced from solar energy. Solar fuels can be produced through photochemical (i.e. activation of certain chemical reactions by photons), photobiological (i.e., artificial photosynthesis), thermochemical (i.e., through the use of solar heat supplied by concentrated solar thermal energy to drive a chemical reaction), and electrochemical reactions (i.e. using the electricity from solar panels to drive a chemical reaction). Light is used as an energy source, with solar energy being transduced to chemical energy, typically by reducing protons to hydrogen, or carbon dioxide to organic compounds. A solar fuel can be produced and stored for later use, when sunlight is not available, making it an alternative to fossil fuels and batteries. Examples of such fuels are hydrogen, ammonia, and hydrazine. Diverse photocatalysts are being developed to carry these reactions in a sustainable, environmentally friendly way. Overview The world's dependen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthracene Photodimerisation
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation. Occurrence and production Coal tar, which contains around 1.5% anthracene, remains a major source of this material. Common impurities are phenanthrene and carbazole. The mineral form of anthracene is called freitalite and is related to a coal deposit. A classic laboratory method for the preparation of anthracene is by cyclodehydration of o-methyl- or o-methylene-substituted diarylketones in the so-called Elbs reaction, for example from ''o''-tolyl phenyl ketone. Reactions Reduction Reduction of anthracene with alkali metals yields the deeply colored radical anion salts M+ nthracenesup>− (M = Li, Na, K). Hydrogenation gives 9,10-dihydroanthrace ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
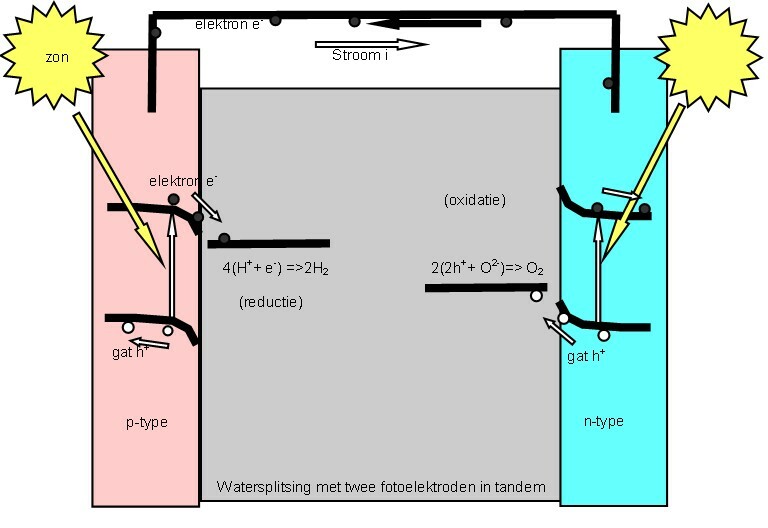
Photoelectrochemical Cell
A "photoelectrochemical cell" is one of two distinct classes of device. The first produces electrical energy similarly to a dye-sensitized photovoltaic cell, which meets the standard definition of a photovoltaic cell. The second is a photoelectrolytic cell, that is, a device which uses light incident on a photosensitizer, semiconductor, or aqueous metal immersed in an electrolytic solution to directly cause a chemical reaction, for example to produce hydrogen via the electrolysis of water. Both types of device are varieties of solar cell, in that a photoelectrochemical cell's function is to use the photoelectric effect (or, very similarly, the photovoltaic effect) to convert electromagnetic radiation (typically sunlight) either directly into electrical power, or into something which can itself be easily used to produce electrical power (hydrogen, for example, can be burned to create electrical power, see photohydrogen). Two principles The standard photovoltaic effect, as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |