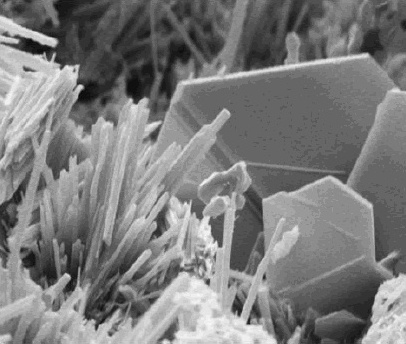
|
Sodium Ferrocyanide
Sodium ferrocyanide is the sodium salt of the coordination compound of formula e(CN)6sup>4−. In its hydrous form, Na4Fe(CN)6 (sodium ferrocyanide decahydrate), it is sometimes known as yellow prussiate of soda. It is a yellow crystalline solid that is soluble in water and insoluble in alcohol. The yellow color is the color of ferrocyanide anion. Despite the presence of the cyanide ligands, sodium ferrocyanide has low toxicity (acceptable daily intake 0–0.025 mg/kg body weight). The ferrocyanides are less toxic than many salts of cyanide, because they tend not to release free cyanide. However, like all ferrocyanide salt solutions, addition of an acid can result in the production of hydrogen cyanide gas, which is extremely toxic. Uses When combined with an Fe(III) salt, it converts to a deep blue pigment called Prussian blue, Fe Cyanide.html"_;"title="e(Cyanide">CN)_It_is_used_as_a_stabilizer_for_the_coating_on_welding.html" ;"title="Cyanide">CN).html" ;"title="Cyanid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. Note that the length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal classes The table below org ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prussian Blue
Prussian blue (also known as Berlin blue, Brandenburg blue or, in painting, Parisian or Paris blue) is a dark blue pigment produced by oxidation of ferrous ferrocyanide salts. It has the chemical formula Fe CN)">Cyanide.html" ;"title="e(Cyanide">CN) Turnbull's blue is chemically identical, but is made from different reagents, and its slightly different color stems from different impurities and particle sizes. Prussian blue was the first modern synthetic pigment. It is prepared as a very fine colloidal dispersion, because the compound is not soluble in water. It contains variable amounts of other ions and its appearance depends sensitively on the size of the colloidal particles. The pigment is used in paints, and it is the traditional "blue" in blueprints, and became prominent in 19th-century () Japanese woodblock prints. In medicine, orally administered Prussian blue is used as an antidote for certain kinds of heavy metal poisoning, e.g., by thallium(I) and radioactive is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Compounds
Sodium atoms have 11 electrons, one more than the stable configuration of the noble gas neon. As a result, sodium usually forms ionic compounds involving the Na+ cation. Sodium is a reactive alkali metal and is much more stable in ionic compounds. It can also form intermetallic compounds and organosodium compounds. Sodium compounds are often soluble in water. Metallic sodium Metallic sodium is generally less reactive than potassium and more reactive than lithium. Sodium metal is highly reducing, with the standard reduction potential for the Na+/Na couple being −2.71 volts, though potassium and lithium have even more negative potentials. The thermal, fluidic, chemical, and nuclear properties of molten sodium metal have caused it to be one of the main coolants of choice for the fast breeder reactor. Such nuclear reactors are seen as a crucial step for the production of clean energy. Salts and oxides Sodium compounds are of immense commercial importance, being particularly centra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Carbonate
Sodium carbonate, , (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield moderately alkaline solutions in water. Historically, it was extracted from the ashes of plants growing in sodium-rich soils. Because the ashes of these sodium-rich plants were noticeably different from ashes of wood (once used to produce potash), sodium carbonate became known as "soda ash". It is produced in large quantities from sodium chloride and limestone by the Solvay process. Hydrates Sodium carbonate is obtained as three hydrates and as the anhydrous salt: * sodium carbonate decahydrate (natron), Na2CO3·10H2O, which readily efflorescence, effloresces to form the monohydrate. * sodium carbonate heptahydrate (not known in mineral form), Na2CO3·7H2O. * sodium carbonate monohydrate (thermonatrite), Na2CO3·H2O. Also known as crystal carbonate. * anhydrous sodium ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Calcium Hydroxide
Calcium hydroxide (traditionally called slaked lime) is an inorganic compound with the chemical formula Ca( OH)2. It is a colorless crystal or white powder and is produced when quicklime (calcium oxide) is mixed or slaked with water. It has many names including hydrated lime, caustic lime, builders' lime, slaked lime, cal, and pickling lime. Calcium hydroxide is used in many applications, including food preparation, where it has been identified as E number E526. Limewater, also called milk of lime, is the common name for a saturated solution of calcium hydroxide. Properties Calcium hydroxide is poorly soluble in water, with a retrograde solubility increasing from 0.66 g/L at 100 °C to 1.89 g/L at 0 °C. With a solubility product ''K''sp of 5.02 at 25 °C, its dissociation in water is large enough that its solutions are basic according to the following dissolution reaction: : Ca(OH)2 → Ca2+ + 2 OH− At ambient temperature, calcium hydroxide (portlandite) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ferrous Chloride
Iron(II) chloride, also known as ferrous chloride, is the chemical compound of formula FeCl2. It is a paramagnetic solid with a high melting point. The compound is white, but typical samples are often off-white. FeCl2 crystallizes from water as the greenish tetrahydrate, which is the form that is most commonly encountered in commerce and the laboratory. There is also a dihydrate. The compound is highly soluble in water, giving pale green solutions. Production Hydrated forms of ferrous chloride are generated by treatment of wastes from steel production with hydrochloric acid. Such solutions are designated "spent acid," or "pickle liquor" especially when the hydrochloric acid is not completely consumed: :Fe + 2 HCl → FeCl2 + H2 The spent acid requires treatment if it is disposed. Ferrous chloride is used in the manufacturing of ferric chloride. Ferrous chloride can also be used to regenerate hydrochloric acid. It is also a byproduct from titanium production, since so ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
European Food Safety Authority
The European Food Safety Authority (EFSA) is the agency of the European Union (EU) that provides independent scientific advice and communicates on existing and emerging risks associated with the food chain. EFSA was established in February 2002, is based in Parma, Italy, and for 2021 it has a budget of €118.6 million, and a total staff of 542. The work of EFSA covers all matters with a direct or indirect impact on food and feed safety, including animal health and welfare, plant protection and plant health and nutrition. EFSA supports the European Commission, the European Parliament and EU member states in taking effective and timely risk management decisions that ensure the protection of the health of European consumers and the safety of the food and feed chain. EFSA also communicates to the public in an open and transparent way on all matters within its remit. Structure Based on a regulation of 2002, the EFSA is composed of four bodies: * Management Board * Executive Dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Kidney
The kidneys are two reddish-brown bean-shaped organs found in vertebrates. They are located on the left and right in the retroperitoneal space, and in adult humans are about in length. They receive blood from the paired renal arteries; blood exits into the paired renal veins. Each kidney is attached to a ureter, a tube that carries excreted urine to the bladder. The kidney participates in the control of the volume of various body fluids, fluid osmolality, acid–base balance, various electrolyte concentrations, and removal of toxins. Filtration occurs in the glomerulus: one-fifth of the blood volume that enters the kidneys is filtered. Examples of substances reabsorbed are solute-free water, sodium, bicarbonate, glucose, and amino acids. Examples of substances secreted are hydrogen, ammonium, potassium and uric acid. The nephron is the structural and functional unit of the kidney. Each adult human kidney contains around 1 million nephrons, while a mouse kidney contains on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anticaking Agents
An anticaking agent is an additive placed in powdered or granulated materials, such as table salt or confectioneries, to prevent the formation of lumps (caking) and for easing packaging, transport, flowability, and consumption. Caking mechanisms depend on the nature of the material. Crystalline solids often cake by formation of liquid bridge and subsequent fusion of microcrystals. Amorphous materials can cake by glass transitions and changes in viscosity. Polymorphic phase transitions can also induce caking. Some anticaking agents function by absorbing excess moisture or by coating particles and making them water-repellent. Calcium silicate (CaSiO3), a commonly used anti-caking agent, added to e.g. table salt, absorbs both water and oil. Anticaking agents are also used in non-food items such as road salt, fertilisers, cosmetics, and detergents. Some studies suggest that anticaking agents may have a negative effect on the nutritional content of food; one such study indicated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Salt Substitute
A salt substitute, also known as low-sodium salt, is a low-sodium alternative to edible salt (table salt) marketed to circumvent the risk of high blood pressure and cardiovascular disease associated with a high intake of sodium chloride while maintaining a similar taste. The leading salt substitutes are non-sodium table salts, which have their tastes as a result of compounds other than sodium chloride. Non-sodium salts reduce daily sodium intake and reduce the health effects of this element. Low sodium diet According to current WHO guidelines, adults should consume less than 2,000 mg of sodium per day (i.e. about 5 grams of traditional table salt), and at least 3,510 mg of potassium per day. In Europe, adults and children consume about twice as much traditional salt as recommended by experts. Examples Potassium Potassium closely resembles the saltiness of sodium. In practice, potassium chloride (also known as potassium salt) is the most commonly used salt subs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Table Salt
Salt is a mineral composed primarily of sodium chloride (NaCl), a chemical compound belonging to the larger class of salts; salt in the form of a natural crystalline mineral is known as rock salt or halite. Salt is present in vast quantities in seawater. The open ocean has about of solids per liter of sea water, a salinity of 3.5%. Salt is essential for life in general, and saltiness is one of the basic human tastes. Salt is one of the oldest and most ubiquitous food seasonings, and is known to uniformly improve the taste perception of food, including otherwise unpalatable food. Salting, brining, and pickling are also ancient and important methods of food preservation. Some of the earliest evidence of salt processing dates to around 6,000 BC, when people living in the area of present-day Romania boiled spring water to extract salts; a salt-works in China dates to approximately the same period. Salt was also prized by the ancient Hebrews, Greeks, Romans, Byzantines, Hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercaptan
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl group, or a sulfanyl group. Thiols are the sulfur analogue of alcohols (that is, sulfur takes the place of oxygen in the hydroxyl () group of an alcohol), and the word is a blend of "''thio-''" with "alcohol". Many thiols have strong odors resembling that of garlic or rotten eggs. Thiols are used as odorants to assist in the detection of natural gas (which in pure form is odorless), and the "smell of natural gas" is due to the smell of the thiol used as the odorant. Thiols are sometimes referred to as mercaptans () or mercapto compounds, a term introduced in 1832 by William Christopher Zeise and is derived from the Latin ('capturing mercury')''Oxford American Dictionaries'' (Mac OS X Leopard). because the thiolate group () bonds very strong ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |